New AB Nexus grant awards spotlight productive cross-campus collaborations
AB Nexus since December 2020
37 research teams awarded grants
$2.5M in total seed funding
39 different areas/departments represented
$13M in external funding awarded
21 publications by awardees
The intercampus program stimulates innovative research collaborations and progress toward solving some of our world’s most pressing health problems.After receiving funding from the AB Nexus grant program, awarded teams have attracted $13 million in outside funding and published more than 20 peer-reviewed research studies.
Today, AB Nexus announced its fifth round of grant awards to researchers from the University of Colorado Anschutz Medical Campus and the University of Colorado Boulder. These teams are comprised of experts from a range of disciplines to advance basic science and translational research that improves human health and well-being, from taking on the most complex forms of cancer to exploring unexpected relationships between periodontal disease and stroke.
AB Nexus, created in 2020 through the University of Colorado President’s Initiative, represents a strategic commitment and financial investment to encourage and expand intercampus research collaborations. Since launching just over two years ago, the initiative has awarded more than $2.5 million in grant funding.
“This program connecting our campuses is truly changing our research culture,” said Massimo Ruzzene, acting vice chancellor for research and innovation at CU Boulder. “Outcomes like 26 new cross-campus collaborations receiving funding since 2020 and the outpouring of volunteers to review proposals confirm what we’ve been hearing from our researchers: AB Nexus is unlocking potential across existing areas of strength on both campuses.”
Two and a half years of AB Nexus programming and funding has already shaped a new culture of collaboration between CU Boulder and CU Anschutz while fostering interdisciplinary partnerships among scientists, engineers and physicians from a diverse range of fields. This enhanced campus connectivity has expanded CU’s collective research enterprise while creating new funding opportunities. Teams funded by AB Nexus have already garnered more than $13 million in external funding and published more than 20 peer-reviewed research studies on topics that range from microplastics to sepsis.
“Since AB Nexus launched in 2020, the program has awarded 37 teams with more than $2.5 million in funding,” said Thomas Flaig, MD, vice chancellor for research at the CU Anschutz Medical Campus. “In a short period of time, this program has demonstrated the value of closer collaboration across the campuses, and we are seeing many novel approaches to better understand and improve human health."
The latest round of AB Nexus awards provides $500,000 across seven teams—five new projects and two that expand upon existing collaborations. These funds include $62,500 from the University of Colorado Cancer Center to co-sponsor cancer-related research projects.
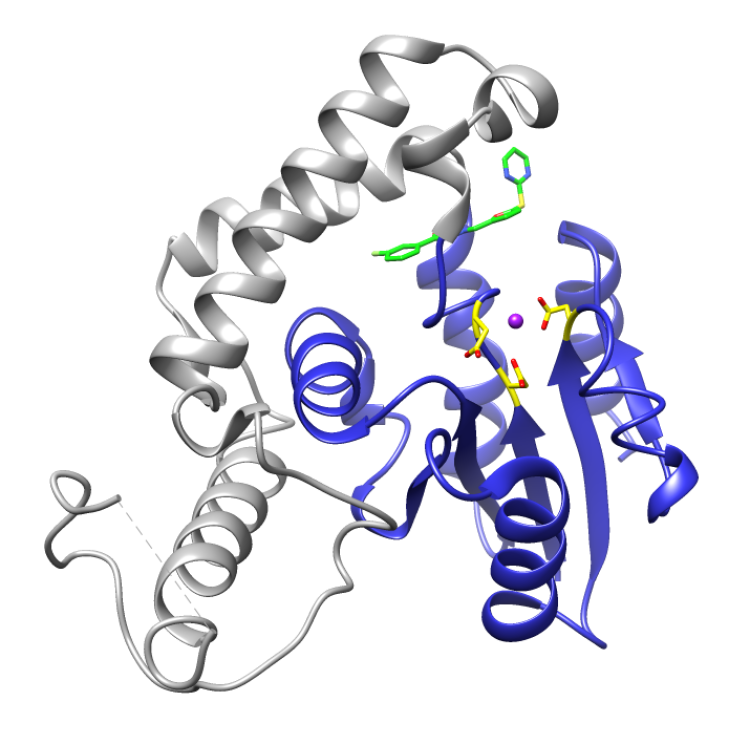
The lead compound (green) binds behind the active site (yellow) of EYA2, disturbing Mg++ binding and inhibiting the enzymatic activity.
Progress toward improving glioblastoma outcomes
One of the most compelling projects awarded this round is led by Heide Ford, professor and CU Medicine endowed chair of pharmacology and the associate director for basic research, University of Colorado Cancer Center, in collaboration with Rui Zhao, professor of biochemistry and molecular genetics at CU Anschutz and Xiang Wang, associate professor of chemistry at CU Boulder. The team is making headway toward developing novel therapeutics targeting glioblastoma (GBM), with a focus on a tyrosine phosphatase, EYA2.
GBM is one of the most aggressive and treatment-resistant cancers, accounting for nearly half of all brain cancers. In spite of the insidious nature of this form of cancer, no approved treatments have significantly improved outcomes for those diagnosed with GBM.
By investigating differences in various cells associated with GBM, the team, in collaboration with Jeremy Rich, MD, professor of neurology at the University of Pittsburgh, was able to identify a gene, EYA2, that is uniquely highly expressed in GBM stem cells and is essential to the success and propagation of malignant growth. With a focus on this important gene, the team is working with a class of lead inhibitors that can target and inhibit these cells and will optimize them toward potent and specific EYA2 phosphatase inhibitors for GBM therapy.

