Chemists find clues to the origins of buckyballs in space

Image taken by the James Webb Space Telescope of the so-called "Pillars of Creation," a region in the Eagle Nebula where clouds of gas and dust are collapsing to form new stars. Credit: NASA, ESA, CSA, STScI; Image Processing: Joseph DePasquale (STScI), Anton Koekemoer (STScI), Alyssa Pagan (STScI)
Far from Earth, in the vast expanses of space between stars, exists a treasure trove of carbon. There, in what scientists call the “interstellar medium,” you can find a wide range of organic molecules—from honeycomblike polycyclic aromatic hydrocarbons (PAHs) to spheres of carbon shaped like soccer balls.
In a new study, an international team of researchers led by scientists at CU Boulder have used experiments on Earth to recreate the chemistry deep in space. The group’s results may have uncovered key steps in the processes that shape these organic molecules over time.

Jordy Bouwman
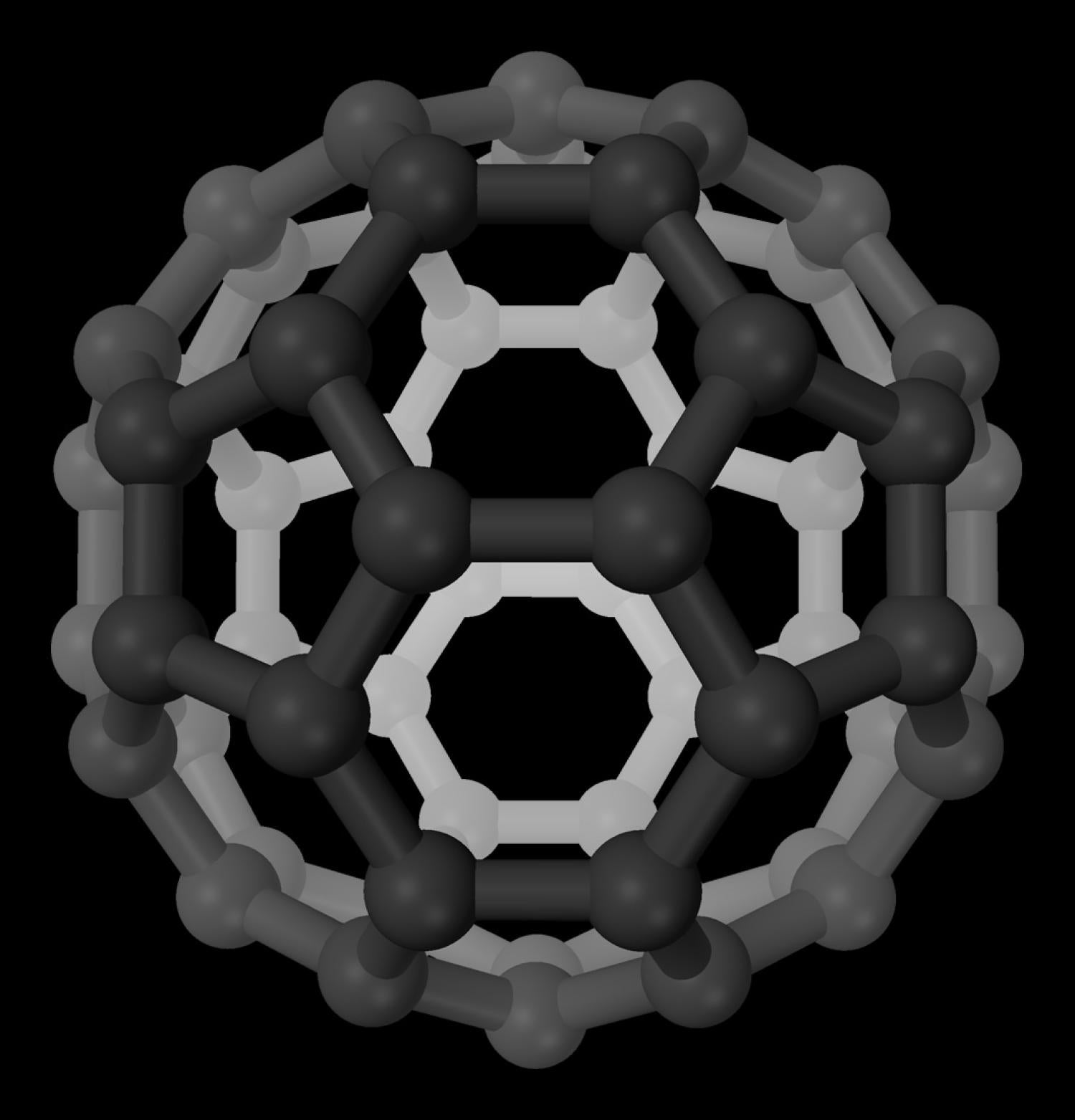
The molecule buckminsterfullerene, shown here, earned its name because it resembles Richard Buckminster Fuller's architectural design for the geodesic dome. (Credit: CC image via Wikimedia commons)
The findings could reveal information about the building blocks that once formed Earth’s solar system, said Jordy Bouwman, lead author of the study. Billions of years ago, similar clouds of matter condensed to form the seeds of what would become our own sun and its planets.
“We’re all made of carbon, so it’s really important to know how carbon in the universe gets transformed on its way to being incorporated in a planetary system like our own solar system,” said Bouwman, an assistant professor at the Department of Chemistry and scientist at the Laboratory for Atmospheric and Space Physics (LASP) at CU Boulder.
The research, published recently in the Journal of the American Chemical Society, sheds light on the formation of a class of molecules called fullerenes.
Fullerenes are made up of carbon atoms organized in the shape of a closed cage. The most famous example is buckminsterfullerene, or the buckyball, which gets its name from famed futurist Richard Buckminster Fuller. These molecules include 60 carbon atoms in the shape of a sphere and bear a striking resemblance to a FIFA regulation soccer ball.
Fullerenes, including buckyballs, float freely in the interstellar medium. But scientists have long struggled to explain where they come from and how they are formed.
The new study suggests that radiation in space may help to transform PAHs into fullerenes.
“This gives us a hint that the buckyballs that we find in space may be connected to these large aromatic molecules that are also abundant,” Bouwman said.
Space chemistry, on Earth
The group simulated the chemistry in space by studying two small PAH molecules called anthracene and phenanthrene.
PAHs are made up of carbon atoms arranged in a series of hexagons, not unlike a honeycomb. They’re abundant on Earth where you can find them in smoke, soot and other charred materials.
“If you put your steak on the grill for too long, and it gets black, that contains PAHs,” Bouwman said. “They’re a nasty byproduct of combustion.”
First, the researchers bombarded the two PAHs with a beam of electrons. It’s similar to what happens when radiation in space interacts with molecules in the interstellar medium.
This bombardment transformed the PAHs into new, charged organic molecules. The researchers then fed the products into an ion trap apparatus at a scientific facility called the Free Electron Lasers for Infrared eXperiments at HFML-FELIX. This one-of-a-kind national research facility is located in Nijmegen in the Netherlands and includes several lasers that spread across a large basement room. Using those lasers, the researchers were able to precisely probe the structure of their new molecules.
They were surprised when they saw the results.

Graphic showing how anthracene, top left, and phenanthrene, bottom right, lose one or two hydrogen atoms to transform into molecules containing carbon atoms in the shape of both hexagons and pentagons. (Credit: Patch et al. 2025, J. Am. Chem. Soc.)
Making buckyballs
Bouwman explained that when the team hit anthracene and phenanthrene with electrons, the molecules lost one or two of their hydrogen atoms.
In the process, they also radically changed their structures, like disassembling a Lego castle and building a new structure. Instead of just including hexagons, the resulting products now carried carbon atoms arranged in the shape of both hexagons and pentagons.
That radical reaction had never been seen before, Bouwman said. Whether these kinds of pentagon-bearing molecules are also common in space isn’t clear.
“That was a very surprising result—that just by kicking off a hydrogen atom or two, the entire molecule completely rearranged,” said Sandra Brünken, a co-author of the study, associate professor at Radboud University in the Netherlands and group leader at FELIX.
The results were eye-opening, in part because those kinds of molecules are also really easy to fold up. (Just picture a soccer ball, which is made up of a mix of both hexagons and pentagons).
In other words, these pentagon-bearing molecules may be the missing link for converting common PAHs into buckyballs and other fullerenes.
Bouwman and Brünken hope that astrophysicists will take note. Scientists could use the team’s findings to see if similar pentagon-bearing molecules exist deep in space using tools like the James Webb Space Telescope—the most powerful telescope ever launched.
“You can take our results from the laboratory, and then use them as a fingerprint to look for the same signatures in space,” Brünken said.
CU Boulder co-authors of the new study include LASP graduate students Madison Patch and Rory McClish. Other co-authors include scientists at Radboud University; Leiden University in the Netherlands; Paris-East Créteil University in France; and the University of Maryland College Park.


