New kinds of liquid crystals resemble solid crystals, could improve computer and TV displays

Solid monoclinic crystals like gypsum are made up of atoms arranged in the shape of a slanted column—what scientists call a "low-symmetry" state. (Credit: Pixabay)
A team at the University of Colorado Boulder has designed new kinds of liquid crystals that mirror the complex structures of some solid crystals—a major step forward in building flowing materials that can match the colorful diversity of forms seen in minerals and gems, from lazulite to topaz.
The group’s findings, published today in the journal Nature, may one day lead to new types of smart windows and television or computer displays that can bend and control light like never before.
The results come down to a property of solid crystals that will be familiar to many chemists and gemologists: Symmetry.
Ivan Smalyukh, a professor in the Department of Physics at CU Boulder, explained that scientists categorize all known crystals into seven main classes, plus many more sub-classes—in part based on the “symmetry operations” of their internal atoms. In other words, how many ways can you stick an imaginary mirror inside of a crystal or rotate it and still see the same structure? Think of this classification system as Baskin-Robbins’ 32 flavors but for minerals.
To date, however, scientists haven’t been able to create liquid crystals—flowing materials that are found in most modern display technologies—that come in those same many flavors.
“We know everything about all the possible symmetries of solid crystals that we can make. There are 230 of them,” said Smalyukh, senior author of the new study who is also a fellow of the Renewable and Sustainable Energy Institute (RASEI) at CU Boulder. “When it comes to nematic liquid crystals, the kind in most displays, we only have a few that have been demonstrated so far.”
That is, until now.
In their latest findings, Smalyukh and his colleagues came up with a way to design the first liquid crystals that resemble monoclinic and orthorhombic crystals—two of those seven main classes of solid crystals. The findings, he said, bring a bit more of order to the chaotic world of fluids.
“There are a lot of possible types of liquid crystals, but, so far, very few have been discovered,” Smalyukh said. “That is great news for students because there’s a lot more to find.”
Symmetry in action
To understand symmetry in crystals, first picture your body. If you place a giant mirror running down the middle of your face, you’ll see a reflection that looks (more or less) like the same person.
Solid crystals have similar properties. Cubic crystals, which include diamonds and pyrite, for example, are made up of atoms arranged in the shape of a perfect cube. They have a lot of symmetry operations.
“If you rotate those crystals by 90 or 180 degrees around many special axes, for example, all of the atoms stay in the right places,” Smalyukh said.
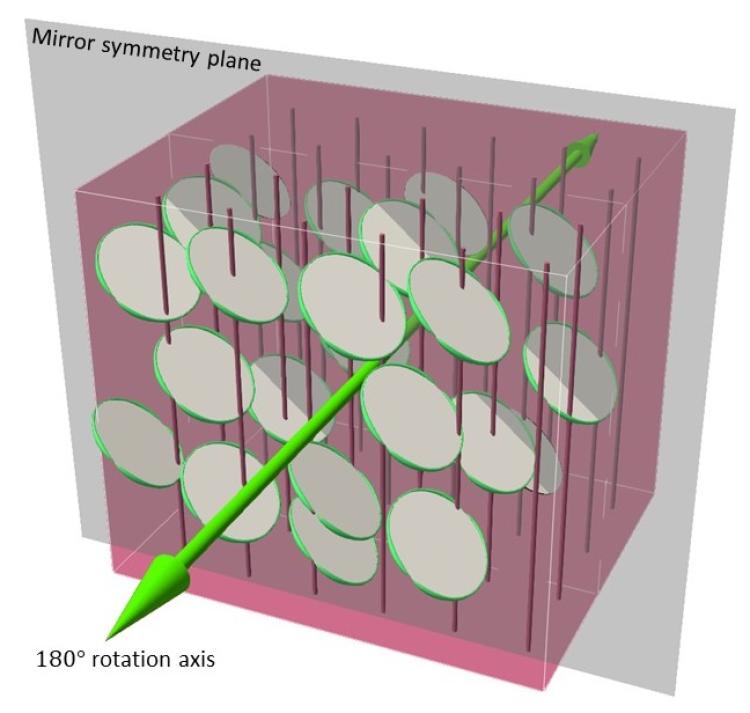
But there are other types of crystals, too. The atoms inside monoclinic crystals, which include gypsum or lazulite, are arranged in a shape that looks like a slanted column. Flip or rotate these crystals all you want, and they still have only two distinct symmetries—one mirror plane and one axis of 180-degree rotation, or the symmetry that you can see by spinning a crystal around an axis and noticing that it looks the same every 180 degrees. Scientists call that a “low-symmetry” state.
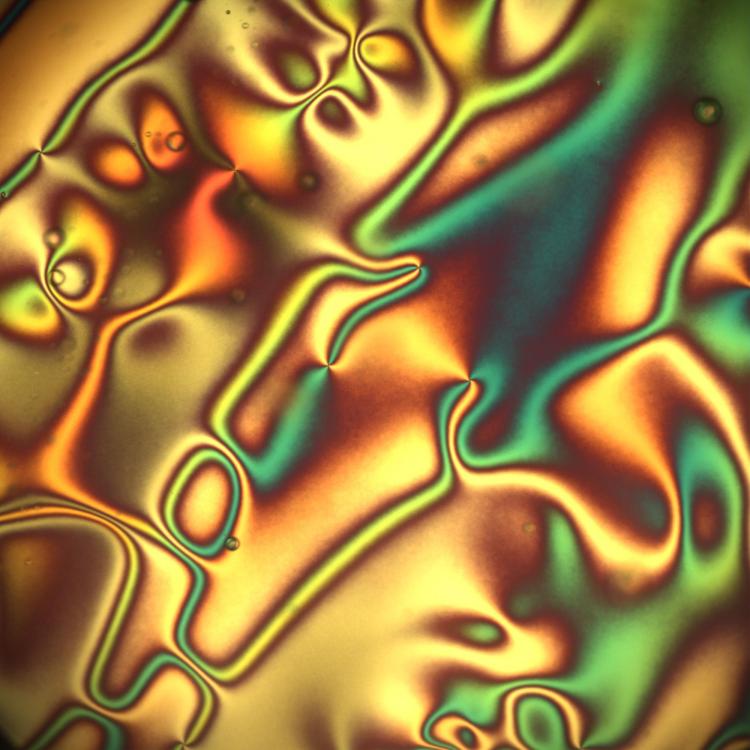
Traditional liquid crystals, however, don’t display those kinds of complex structures. The most common liquid crystals, for example, are made up of tiny rod-shaped molecules. Under the microscope, they tend to line up like dry pasta noodles tossed into a pot, Smalyukh said.
“When things can flow they don’t usually exhibit such low symmetries,” Smalyukh said.
Order in liquids
He and his colleagues wanted to see if they could change that. To begin, the team mixed together two different kinds of liquid crystals. The first was the common class made up of rod-shaped molecules. The second was made up of particles shaped like ultra-thin disks.
When the researchers brought them together, they noticed something strange: Under the right conditions in the lab, those two types of crystals pushed and squeezed each other, changing their orientation and arrangement. The end result was a nematic liquid crystal fluid with symmetry that looks a lot like that of a solid monoclinic crystal. The molecules inside displayed some symmetry, but only one mirror plane and one axis of 180-degree rotation.
The group had created, in other words, a material with the mathematical properties of a lazulite or gypsum crystal—but theirs could flow like a fluid.
“We’re asking a very fundamental question: What are the ways that you can combine order and fluidity in a single material?” Smalyukh said.
And, the team’s creations are dynamic: If you heat the liquid crystals up or cool them down, for example, you can morph them into a rainbow of different structures, each with their own properties, said Haridas Mundoor, lead author of the new paper. That’s pretty handy for engineers.
“This offers different avenues that can modify display technologies, which may enhance the energy efficiency in performance of devices like smart phones,” said Mundoor, a postdoctoral research associate at CU Boulder.
He and his colleagues are still nowhere near making liquid crystals that can replicate the full spectrum of solid crystals. But the new paper gets them closer than ever before—good news for fans of shiny things everywhere.
Other coauthors on the new paper include Jin-Sheng (Jason) Wu, a graduate student at CU Boulder, and Henricus Wensink of the Université Paris-Saclay.