Francisco Apen - Project Profile
Ph.D. Candidate, University of California-Santa Barbara, during AGeS project
…and Apatite for All: Characterizing new reference materials for apatite petrochronology
Apatite [formula: Ca5(PO4)3(F, Cl, OH)] is a mineral we should all be familiar with—our teeth are made of the OH-endmember apatite! For geologists, apatite is a well-known mineral because it commonly occurs in sedimentary, igneous, and metamorphic rocks. From a geochemical perspective, apatite is a handy mineral because it can host substantial amounts of U and other incompatible elements (like Sr, Y, and the rare-earth elements), such that it can be used as a U-Th-Pb geochronometer, Sr and Nd isotope tracer of petrogenetic processes, and monitor of the volatile evolution of magmas. Microbeam analytical methods (such as Laser Ablation Inductively Coupled Plasma Mass Spectrometry [LA-ICP-MS]) provide high-spatial resolution data that in turn allow detailed studies of apatite geochemistry.

One of the apatite specimens.
Apatite commonly has a hexagonal
prism crystal habit like the one shown here.
This crystal is roughly 1 inch in height.
Apatite [formula: Ca5(PO4)3(F, Cl, OH)] is a mineral we should all be familiar with—our teeth are made of the OH-endmember apatite! For geologists, apatite is a well-known mineral because it commonly occurs in sedimentary, igneous, and metamorphic rocks. From a geochemical perspective, apatite is a handy mineral because it can host substantial amounts of U and other incompatible elements (like Sr, Y, and the rare-earth elements), such that it can be used as a U-Th-Pb geochronometer, Sr and Nd isotope tracer of petrogenetic processes, and monitor of the volatile evolution of magmas. Microbeam analytical methods (such as Laser Ablation Inductively Coupled Plasma Mass Spectrometry [LA-ICP-MS]) provide high-spatial resolution data that in turn allow detailed studies of apatite geochemistry.

Pilot LA-ICP-MS data from the apatite specimens done at UC Santa Barbara yielded homogeneous U-Pb ages and high concentrations of incompatible elements. While these data suggested that the apatites could be suitable RMs, they are calculated with respect to an apatite primary standard and their uncertainties are limited to the ~2% based on the external reproducibility of the UCSB lab. To independently verify and establish the U-Pb, Sm-Nd, and Sr isotopic compositions of these RMs, I undertook Isotope Dilution Thermal Ionization Mass Spectrometry (ID-TIMS) at the Boise State University Isotope Geology Laboratory under the tutelage of Drs. Mark Schmitz and Corey Wall. In contrast to LA-ICP-MS, ID-TIMS involves dissolving crystal fragments and separating elements of interest through anion exchange chromatography.

Once purified, the dissolved aliquots were loaded onto metal filaments that were then placed into a TIMS instrument and gradually heated up to release the elements of interest into the mass spectrometer. Preparations and analyses for TIMS did take significantly longer compared to LA-ICP-Ms (days vs. hours), but the end results from the TIMS analyses were very precise determinations of the isotopic compositions of the apatite RMs.

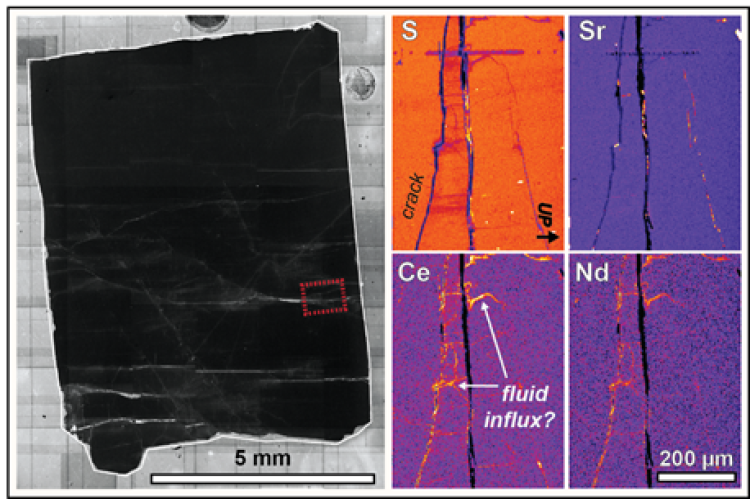
The ID-TIMS analyses support use of one apatite specimen as a suitable RM, but the analyses also revealed heterogeneity in another apatite specimen. I suspect that chemical heterogeneity is related to fine-grained features within the crystals—likely having formed when fluids intruded into the apatite along cracks. Continued chemical mapping and imaging of the crystals should help guide how to best deal with this small-scale heterogeneity and optimize the specimen as a useful RM. Ultimately, with the TIMS and LA-ICP-MS data at hand, I hope to provide the geochronological community new materials to help further exploit apatite as an insightful geochemical tracer and also facilitate inter-laboratory comparisons.
Check out my webpage to learn about my other projects: https://sites.google.com/view/apen-earth

