Crystallization for Water Treatment
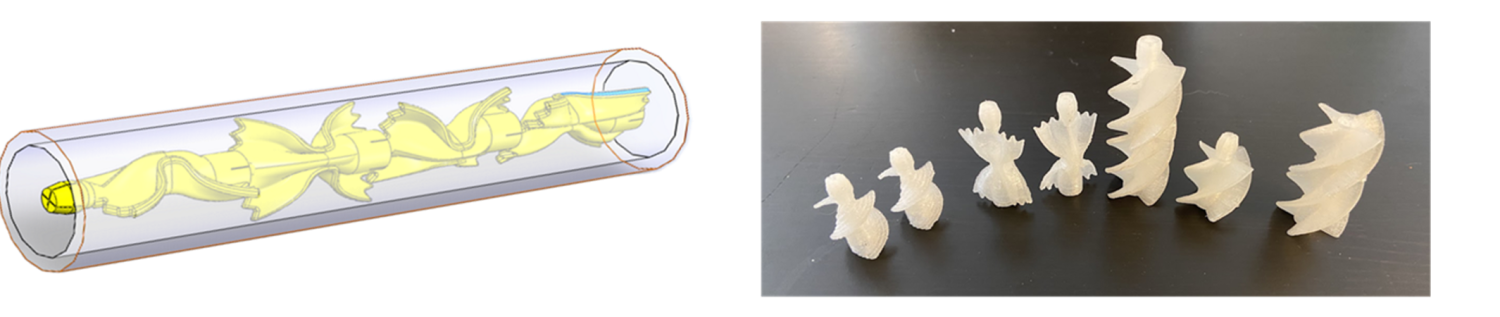
Waters supersaturated with sparingly soluble salts are encountered in RO/NF (reverse osmosis/nanofiltration) membrane-based water treatment processes, cooling water discharge, and produced water from oil and natural gas operations. Thermodynamically driven de-supersaturation results in salt precipitation causing strongly adherent mineral scale formation on equipment surfaces, this is responsible for plumbing blockage, decreased efficiency of heat transfer and separation operations (membrane scaling), etc. Moreover recovering useable water from supersaturated brines generated as reject streams from RO/NF process can improve overall process efficiency. Therefore a good understanding of crystallization can aid in developing more efficient process engineering approaches. With the aforementioned aims, we have studied crystallization kinetics of supersaturated brines using an MSMPR-in-Series bench scale setup under steady-state conditions. We are currently working on designing Static Mixers that can function as inline crystallizers to treat supersaturated brines.
We are also studying the relationship between the surface chemistry of materials and their propensity to suffer from mineral scaling. Understanding the relationship between surfaces and crystallization would aid in developing RO/NF membranes that are less prone to mineral scaling and in general, materials that can efficiently function in environments requiring the handling of supersaturated electrolytes. We have also looked at the effect of engineered micron and sub-micron scale features embossed on membranes and, polymer surfaces in terms of mineral scaling when exposed to supersaturated waters.
Graduate Students
Chris Buelke chbu4440@colorado.edu
Sankar A. Ravi Sankar.Ravi@colorado.edu
[video:https://youtu.be/hc1Yx9tIO7A]
Patterning Membranes

Pasta Inspired Static Mixer for Crystallization
Static Mixer Operation
[video:https://youtu.be/v-UU06-5xSU]
Acknowledgements


