Small Molecule Regulation of CRISPR-Cas9 Using RNA Aptamers
Background
CRISPR-Cas9 has led to great advances in gene editing for a broad spectrum of applications. To further the utility of Cas9, there have been efforts to achieve temporal control over its nuclease activity. While different approaches have focused on regulation of Cas9 or gRNA-regulated CRISPR interference, none of the reported methods enable stringent control of the nuclease activity in bacteria. As a result, there remains a need for improved methods to temporally control Cas9 activity. Currently CRISPR-based editing technologies suffers from low transformation efficiencies caused by the lethality of dsDNA breaks in bacteria and from associated issues, such as biases in multiplexed libraries towards non-cutting gRNAs.
Technology
Researchers at CU Boulder, led by Biochemistry Professor Robert Batey, have developed compositions and methods for temporal regulation of single guide RNAs (sgRNAs) that comprise a small molecule-binding aptamer in the sgRNA, which enables small-molecule-dependent gene editing in bacteria. They also developed a method for selecting sgRNAs that are dependent on other small molecules of interest.
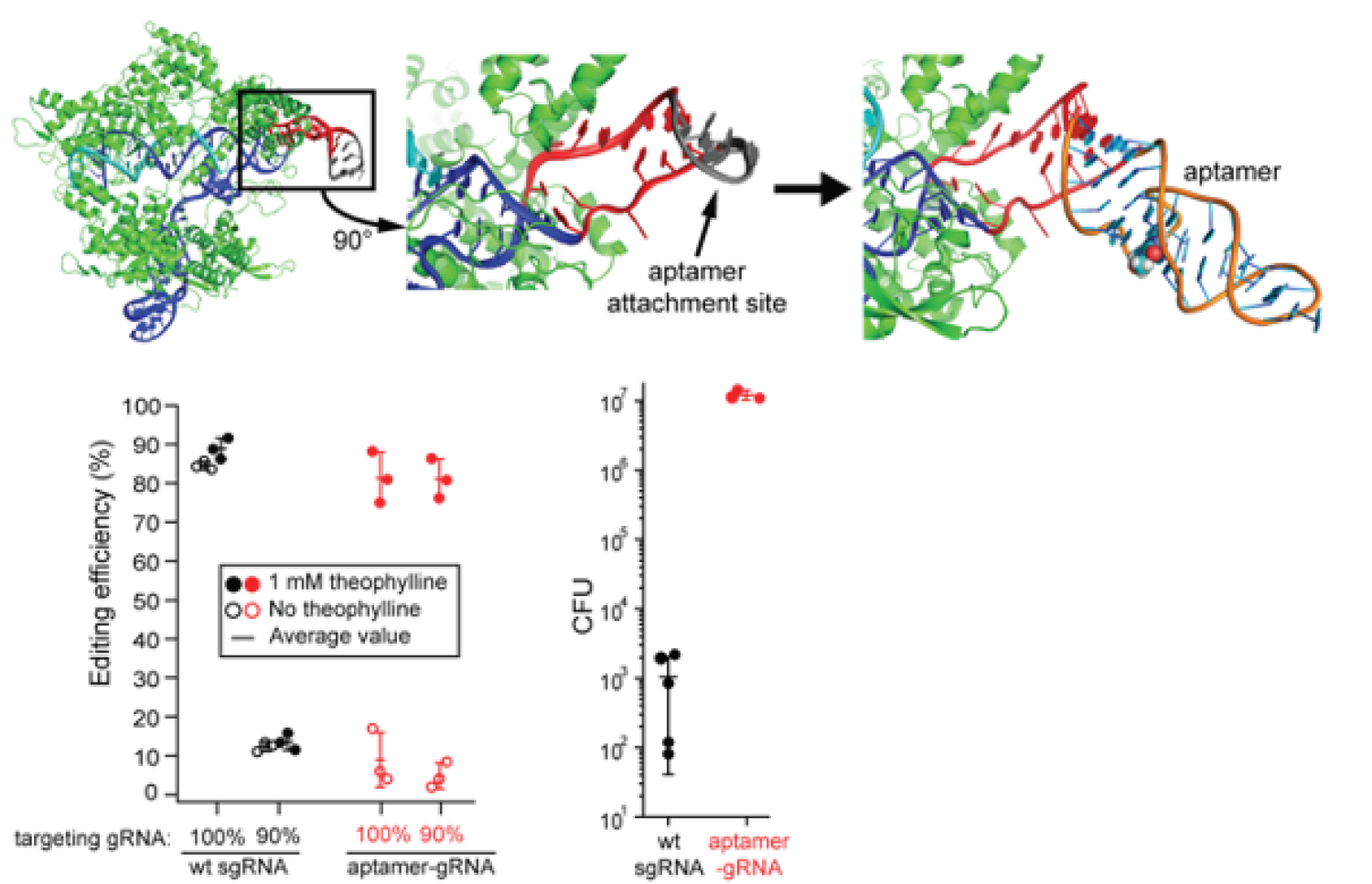
Figure (Top): The aptamer is inserted into the RNA loop (gray) and binding by the theophylline molecule (spheres) is transmitted to the gRNA (blue )via the RNA linker (red).
Figure (Bottom Left): Escapees outgrow edited bacteria and apparent editing efficiency drops when 10% non-targeting gRNA are co-transformed. Editing with aptamer-gRNAs alleviates this issue.
Figure (Bottom Right): Inducing gene editing with aptamer-gRNAs increases the CFU of transformants without compromising editing efficiency.
Advantages
Here, novel RNA linkers were used to combine theophylline-and 3-methylxanthine (3MX) binding aptamers with the gRNA, enabling small molecule-dependent editing in Escherichia coli. These activatable guide RNAs enable orthogonal, temporal and post-transcriptional control of in vivo gene editing.
Further, they reduce the death of host cells caused by cuts in the genome, a major limitation of CRISPR-mediated bacterial recombineering. Temporal control of the sgRNAs will allow timing and titration of the nuclease activity to simultaneously increase transformation efficiency and decrease bias towards unedited escapee cells.
What's Next?
This technology is available for non-exclusive licensing.
Nicole Forsberg: nicole.forsberg@colorado.edu
The Newsroom
For marketing and communication inquiries or news tips, contact Daniel Leonard, senior marketing and communications specialist for Venture Partners at CU Boulder.
For media inquiries, please visit colorado.edu/news/formedia.


