Chemical Kinetics
Introduction
At the high temperatures encountered by hypersonic vehicles, gasses chemically react. Room temperature air is mostly N2 and O2, but around 3000 K oxygen starts to dissociate, or break down into its constitutent atoms. Nitrogen, which has a stronger bond, dissociates starting around 6000 K. These processes have a profound effect on the gas environment surrounding hypersonic vehicle, for several reasons. First, dissociating a molecule takes energy, and so the gas cools as it dissociates, decreasing the heating experienced by vehicles in flight. Second, breaking one molecule into two atoms increases the pressure, potentially affecting the shock shape and vehicle aerodynamics. Finally, different chemicals interact differently with surfaces; atomic oxygen in particular is highly reactive with carbon-based heat shields (see also the section on Material Response & Ablation). Therefore, we must be able to predict the chemical evolution of a gas at high temperatures.
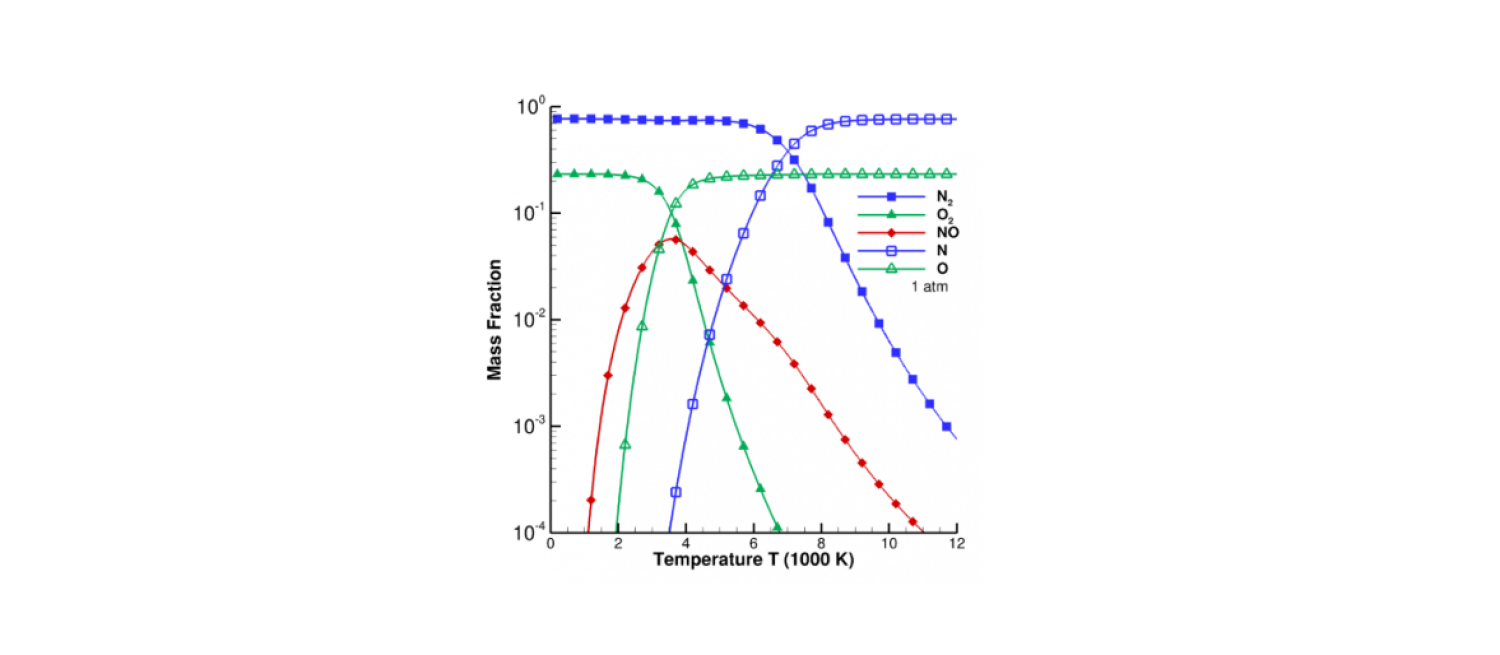
Mass fractions of air at chemical equilibrium for 1 atmosphere, neglecting ionization
Nonequilibrium Chemical Kinetics
A hot gas dissociates very quickly, on the order of microseconds. One might think we could neglect the speed of dissociation, then, and model the gas in an equilibrium state. However, many things happen very quickly in hypersonic flows, and so the speed of reaction often matters. For example, suppose that the air behind a strong shock is very hot and travelling at 1 km/s. If dissociation takes 10 μs, then the gas has travelled 1 cm in the time it took to dissociate. If 1 cm is the distance from the shock to the vehicle surface, then the state of the gas as it hits the surface depends on how quickly it dissociates. Of course, this is a simplified example, and other processes such as recombination and stagnation would be important. Nevertheless, many conditions encountered in hypersonic flows require an understanding of the finite rate at which chemical reactions occur: the chemical kinetics of high-temperature flows.
Research at NGPDL
We are examining the vehicle geometries and freestream conditions where quantities of interest, such as heat flux, are particularly sensitive to chemical kinetics. After all, not all cases depend on the reaction rate: the density of low-altitude flight (less than about 10 km) means there are so many collisions that the gas comes to chemical equilibrium very quickly. Similarly, very high-altitude flight (above about 70 km) has so few collisions that essentially no chemical reactions occur regardless of their reaction rate. Between these extremes, chemical kinetics typically matter, but some altitudes more than others. Understanding which conditions are the most sensitive, and why, is critical for validating new chemical kinetics models.
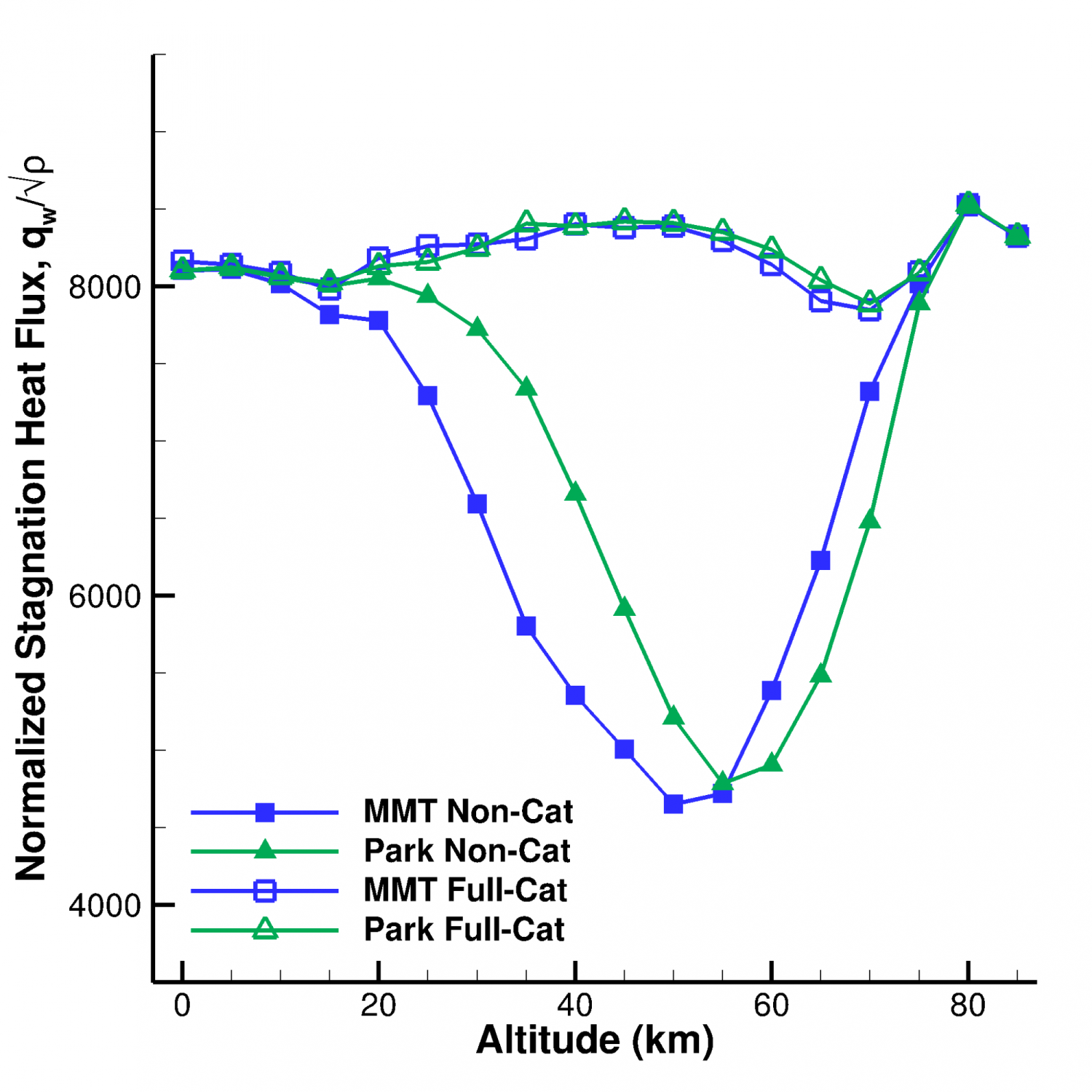
Normalized stagnation point heat flux as a function of freestream altitude, for several chemical kinetics models, from Ref. [1].
We are also collaborating with the Hanson group at Stanford, who are using shock tubes to accurately measure reaction rates. Their cutting-edge experimental results are combined with high fidelity computational models of chemical kinetics here at NGPDL.
Investigators
Acknowledgements
Funding for this work is provided by the Air Force Office of Scientific Research (AFOSR) and Lockheed Martin Corporation.
References
[1] Chaudhry, R. S., Boyd, I. D., and Candler, G. V., “Vehicle-Scale Simulations of Hypersonic Flows using the MMT Chemical Kinetics Model,” AIAA AVIATION 2020 FORUM, AIAA Paper 2020-3272, 2020. doi: 10.2514/6.2020-3272.

