Tuning the Molecular Interaction of Enzymes with Solvents via Polymer Modification
This method uses polymer modifications to tune enzyme activity for increased solubility in non-native solvents.
Background
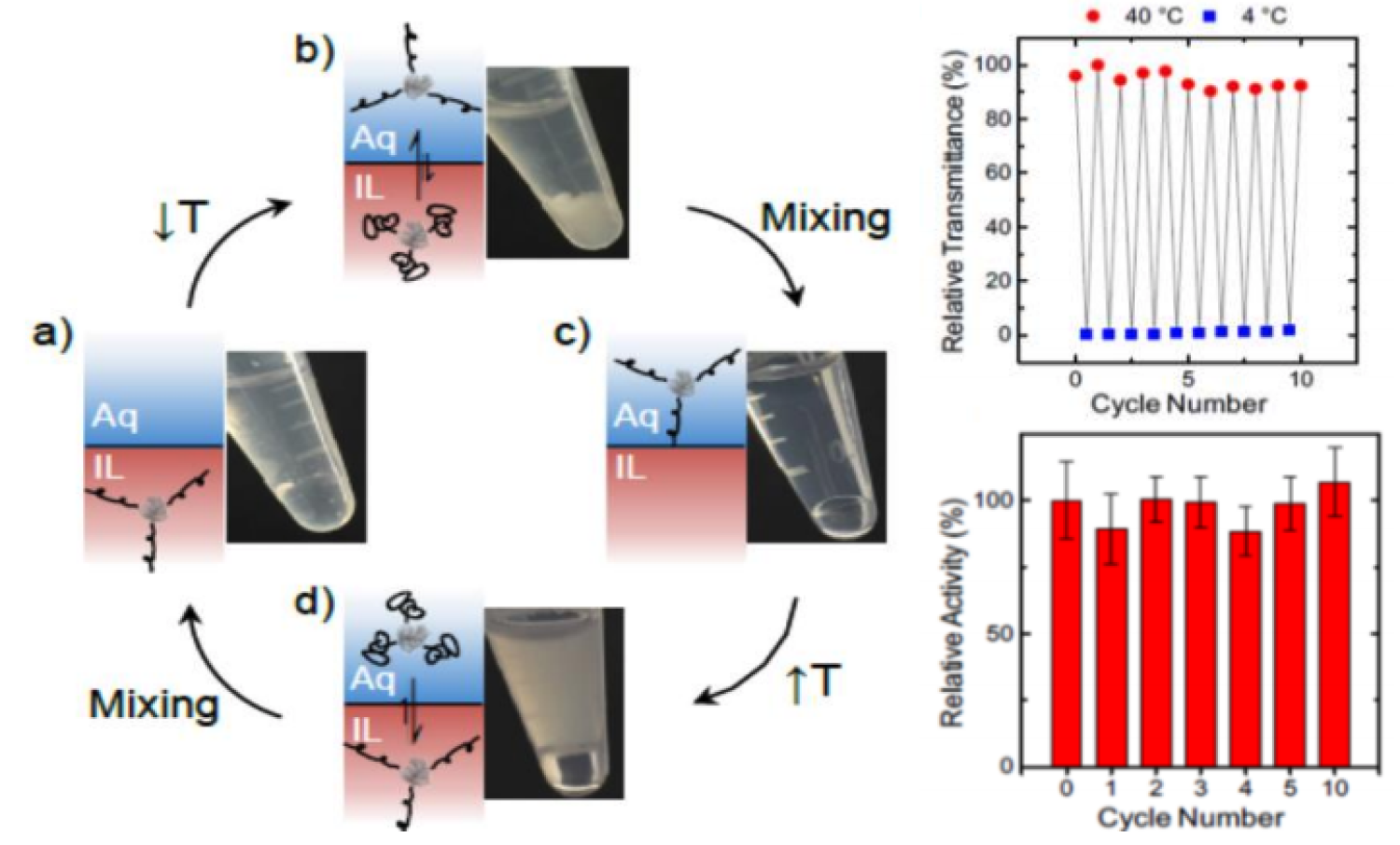
Tuning the miscibility of enzymes in non-native solvent environments has important implications for the use of enzymes in industrial chemical transformations. Traditionally, because the preparation and purification of enzymes can be costly, enzymes are used as heterogeneous catalysts, which facilitates their separation and recycling but can severely impact their activity. One approach to leverage the benefits of heterogeneous and homogeneous biocatalysis entails using responsive materials that permit the miscibility of the enzyme to be altered adaptively. In this approach, the thermodynamic interactions between the enzyme and solvent may be varied via modification with materials that have switchable properties. By modifying an enzyme with such materials, the enzyme may be transferred between heterogeneous and homogeneous states in the reaction solvent. These methods have been demonstrated in aqueous solutions; this work extends these methodologies to non-aqueous (non-native) solvents.
Technology Overview
Researchers at the University of Colorado Boulder have demonstrated the use of polymer modification to specifically modulate the interactions of enzymes with non-native solvents. This approach allows one to rationally tune the interactions of the enzyme-polymer conjugate with the solvent in a thermoresponsive manner within a desired temperature range. By rationally altering the composition of the polymer, the solubility and temperature of the phase separation can be quantitatively controlled and tuned. This approach ultimately permits the advantages of homogeneous (high activity, low diffusional limitations) and heterogeneous (recyclability) biocatalysis in non-native solvents to be exploited.
Key Results
The activity of the enzyme can be tuned by increasing its solubility in a desired solvent. The enzyme can be readily recycled via sequential dissolution and precipitation, without loss of activity. The enzyme can also be reversibly shuttled between a non-aqueous solvent and buffer in response to changes in temperature.
Stage of Development
Proof of Concept.
Benefits
- Dramatic enhancements in activity in non-aqueous media due to increase in solubility.
- Ability to tune the critical phase transition temperature.
- Liquid-liquid extraction.
- Easy recovery of enzyme (e.g., through temperature depending precipitation of liquid-liquid extraction.
Applications
- Biocatalysis
- Bioseparations
- Bionanotechnology
- Drug delivery
Opportunity
Available for exclusive or non-exclusive licensing. CU4825B
Patents
Provisional patent filed. Available upon request.
IP Status
Provisional patent
Seeking
Licensing
Nicole Forsberg: nicole.forsberg@colorado.edu
The Newsroom
For marketing and communication inquiries or news tips, contact Daniel Leonard, senior marketing and communications specialist for Venture Partners at CU Boulder.
For media inquiries, please visit colorado.edu/news/formedia.


