Regulation of RNAPII activity by Transcription Factors, IDRs, condensates
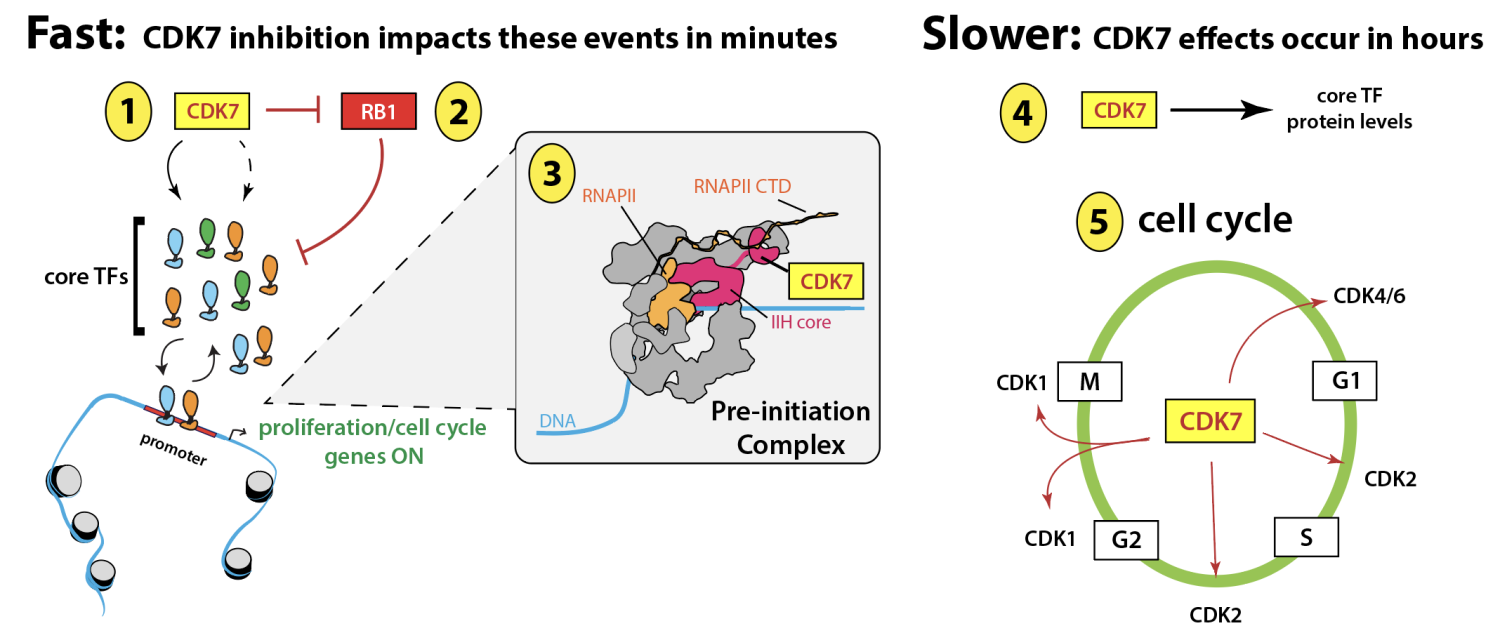
Sequence-specific, DNA-binding TFs are the major regulators of cell identity and cell physiology. As examples, expression of select TFs can revert differentiated cells back to a pluripotent state and mutations in a single TF can drive tumor formation. Notably, sequence-specific DNA-binding TFs represent a major class of targets for the transcription-associated kinases CDK7, CDK8 and its paralog CDK19. Our lab is using a variety of approaches to better understand how TF activity is controlled by kinases. The schematic shown below summarizes a recent study that showed how the TFIIH-associated kinase CDK7 controls the function of a common core set of TFs that drive proliferative gene expression programs across diverse cell types (Jones et al. Sci Adv 2025 eadr9660).
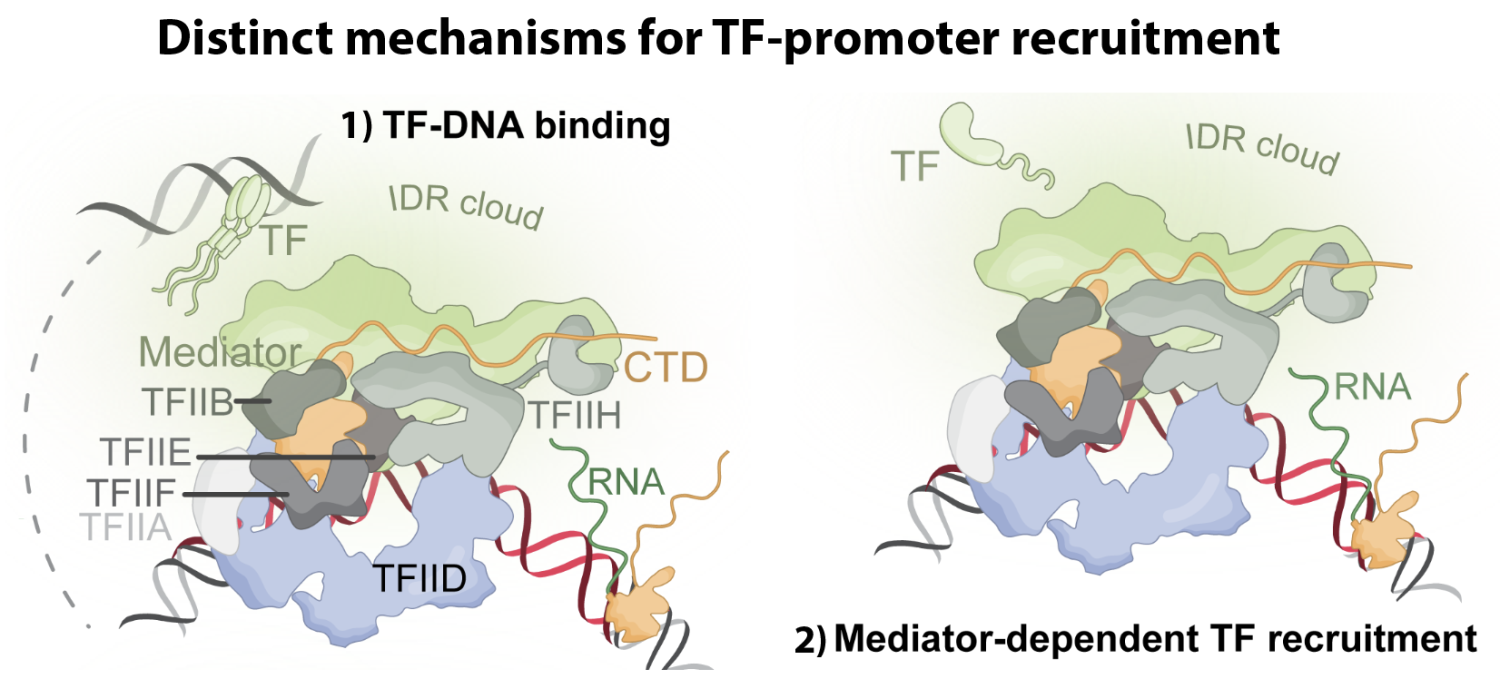
TFs are also enriched in intrinsically disordered regions (IDRs), which contribute to low-affinity interactions with other IDR-containing proteins and enhance TF partitioning in the nucleus. TF partitioning increases local TF concentrations and may promote phase separation/condensate formation. TF IDR sequences commonly overlap with TF activation domains, which directly interact with RNAPII co-regulators (e.g. Mediator) with high affinity. The dual role for IDR-dependent regulation of i) low-affinity partitioning and ii) high-affinity protein-protein interactions creates experimental challenges to de-couple IDR-specific functions. Ongoing projects in the lab focus on the mechanisms by which IDRs regulate RNAPII function and kinetics, condensate formation, and nuclear partitioning, using a combination of biochemical, biophysical, cellular, and computational methods. The schematic below summarizes one of several recent findings from smTIRF experiments, which showed that Mediator is sufficient to recruit TFs to promoters, without TF-DNA binding (Palacio & Taatjes Cell Rep 2025 116251). Notably, we observed that rapid TF-promoter binding and rapid RNAPII activation was dependent on IDRs but not condensate formation per se.
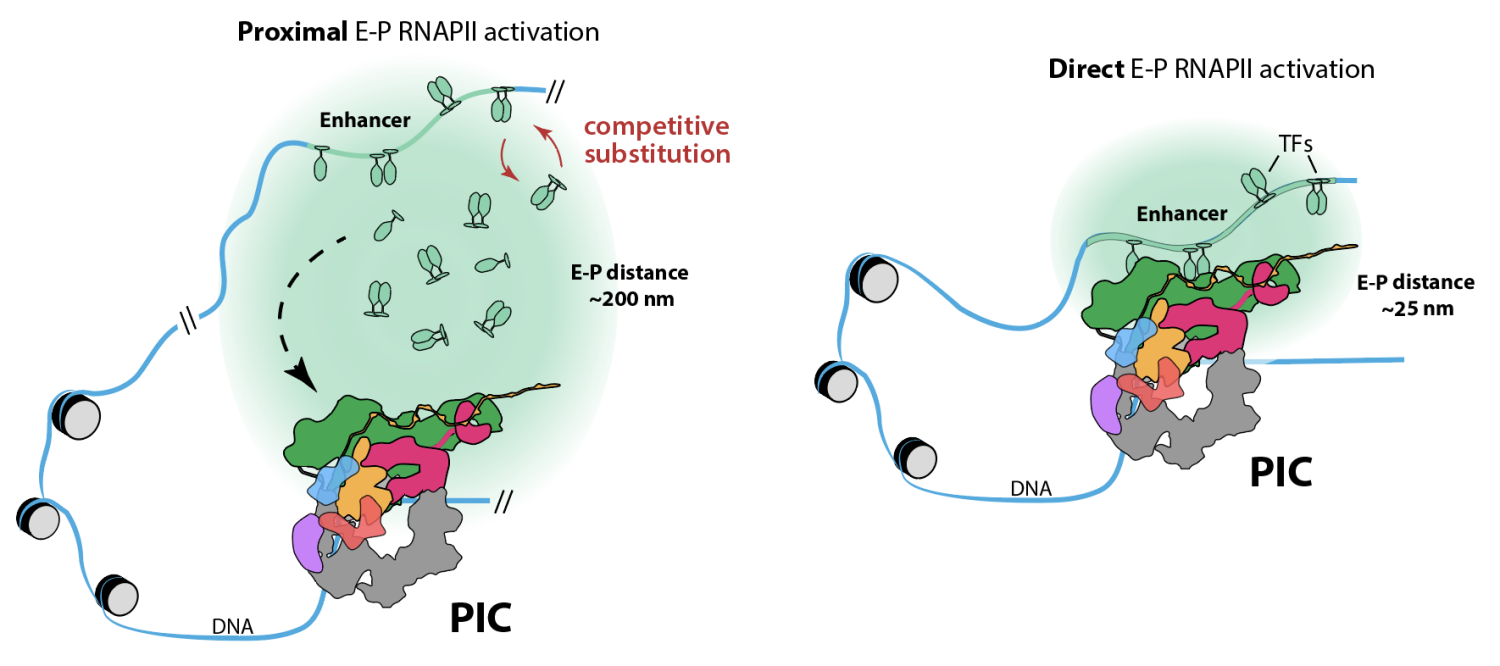
Mediator is a common target of DNA-binding transcription factors (TFs) and also interacts with various components within the Pre-Initiation Complex (PIC), which consists of TFIIA, TFIIB, TFIID, TFIIE, TFIIF, TFIIH, Mediator itself, and RNAPII. As a central integrator of both general and TF-specific regulatory signals, Mediator plays a prominent role in controlling transcription on a genome-wide scale. Because Mediator interacts with both DNA-binding TFs and the general transcription machinery, including RNAPII, Mediator acts as a molecular bridge to link these factors and facilitate TF-dependent regulation of gene expression. A fundamentally important consequence of TF-Mediator interactions is RNAPII activation within the PIC; TF-Mediator interactions may also contribute to formation of enhancer-promoter loops and enhancer-dependent RNAPII activation. The scheme below shows two distinct but not mutually exclusive mechanisms by which enhancers may activate RNAPII at a nearby promoter (from Nagel & Taatjes Mol Cell 2025 1907).
Given their central roles in development and disease, DNA-binding TFs represent quintessential targets for molecular therapeutics. However, TFs are generally considered “undruggable” due to few successes with small molecule compounds, despite large-scale efforts. Because TFs activate transcription through the Mediator complex, the TF-Mediator interface represents a key “control point” for transcription. Through collaborative efforts, we are working to develop chemical probes to target key TF-Mediator interfaces. Although our primary goal is to utilize these probes for detailed mechanistic studies in vitro and in cells, these efforts may help establish Mediator as a viable therapeutic target.
Recent publications related to this topic:
Palacio M; Taatjes DJ. Real-time visualization of reconstituted transcription reveals RNA polymerase II activation mechanisms at single promoters. Cell Rep 2025, 44: 116251.
Nagel, M; Taatjes, DJ. Regulation of RNA polymerase II transcription through re-initiation and bursting. Mol Cell 2025, 85: 1907-1919.
Jones, T; Sigauke, RF; Sanford, L; Taatjes, DJ; Allen, MA; Dowell, RD. TF profiler: a transcription factor inference method that broadly measures transcription factor activity and identifies mechanistically distinct networks. Genome Biol 2025, 26: 92.
Jones, T; Feng, J; Luyties, O; Cozzolino, K; Sanford, L; Rimel, JK; Ebmeier, CC; Shelby, GS; Watts, LP; Rodino, J; Rajagopal, N; Hu, S; Brennan, F; Maas, ZL; Alnemy, S; Richter, WF; Koh, AF; Cronin, NB; Madduri, A; Das, J; Cooper, E; Hamman, KB; Carulli, JP; Allen, MA; Spencer, S; Kotecha, A; Marineau, J; Greber, BJ; Dowell, RD; Taatjes DJ. TFIIH kinase CDK7 drives cell proliferation through a common core transcription factor network. Sci Adv 2025, 11: eadr9660.
Palacio, M; Taatjes, DJ. Transcription regulation through selective partitioning: weak interactions with a strong foundation. Mol Cell 2024, 84: 3375 - 3377
Maia-Silva, D; Cunniff, PJ; Schier, AC; Skopelitis, D; Trousdell, MC; Moresco, P; Gao, Y; Kechejian, V; He, X; Sahin, Y; Wan, L; Alpsoy, A; Liverpool, J; Krainer, AR; Egeblad, M; Spector, DL; Fearon, DT; Dos Santos, CO; Taatjes, DJ; Vakoc, CR. Interaction between MED12 and DNp63 activates basal identity in pancreatic ductal adenocarcinoma. Nat Genet 2024, 56: 1377 - 1385.
Nayak, S; Taatjes DJ. SnapShot: Mediator Complex Structure. Cell 2022, 185: 3458.
Richter, WF; Nayak, S; Iwasa, J; Taatjes, DJ. The Mediator complex as a master regulator of transcription by RNA polymerase II. Nat Rev Mol Cell Biol 2022, 23: 732 - 749.
Palacio, M; Taatjes DJ. Merging established mechanisms with new insights: Condensates, hubs, and the regulation of RNA polymerase II transcription. J Mol Biol 2022, 433: 167216.
Allen, BL; Quach, K; Jones, T; Levandowski, CB; Ebmeier, CC; Rubin, JD; Read, T; Dowell, RD; Schepartz, A; Taatjes, DJ. Suppression of p53 response by targeting p53–Mediator binding with a stapled peptide. Cell Rep 2022, 39: 110630.
Levandowski, CB; Jones, T; Gruca, M; Ramamoorthy, S; Dowell, RD; Taatjes, DJ. The ∆40p53 isoform inhibits p53-dependent eRNA transcription and enables regulation by signal-specific transcription factors during p53 activation. PLoS Biol 2021, 19: e3001364.
Rubin, JD; Stanley, JT; Sigauke, RS; Levandowski, CB; Maas, ZL; Westfall, J; Taatjes, DJ; Dowell, RD. Transcription factor enrichment analysis (TFEA) quantifies the activity of multiple transcription factors from a single experiment. Commun Biol 2021, 4: 661.
Rimel, JK; Poss, ZC; Erickson, B; Maas, ZL; Ebmeier, CC; Johnson, JL; Decker, T-M; Yaron, TM; Bradley, MJ; Hamman, KB; Hu, S; Malojcic, G; Marineau, JJ; White, PW; Brault, M.; Tao, L.; DeRoy, P; Clavette, C; Nayak, S; Damon, LJ; Kaltheuner, IH; Bunch, H; Cantley, LC; Geyer, M; Iwasa, J; Dowell, RD; Bentley, DL; Old WM; Taatjes, DJ. Selective inhibition of CDK7 reveals high-confidence targets and novel mechanisms for TFIIH function in transcription. Genes Dev 2020; 34: 1452 – 1473.
Zamudio, AV; Dall’Agnese, A; Henninger, JE; Manteiga, JC; Afeyan, LK; Hannett, NM; Coffey, EL; Li, CH; Oksuz, O; Boija, A; Klein, IA; Sabari, BR; Hawken, SW; Spille, JH; Decker, TM; Cisse, II; Abraham, BJ; Lee, TI; Taatjes, DJ; Schuijers, J; Young, RA. Mediator condensates localize signaling factors to key cell identity genes. Mol Cell 2019, 76: 753 – 766.
Steinparzer, I; Sedlyarov, V; Rubin, JD; Eislmayr, K; Galbraith MD; Levandowski, CB; Vcelkova, T; Sneezum, L; Wascher, F; Amman, F; Kleinova, R; Bender, H; Andrysik, Z; Espinosa, JM; Superti-Furga, G; Dowell, RD; Taatjes, DJ; Kovarik, P. Transcriptional responses to IFNg require Mediator kinase-dependent pause release and mechanistically distinct CDK8 and CDK19 functions. Mol Cell 2019, 76: 485 – 499.
Guo, YE; Manteiga, JC; Henninger, J; Sabari, BR; Dall'Agnese, A; Hannett, NM; Spille, J-H; Afeyan, LK; Zamudio, AV; Shrinivas, K; Abraham, BJ; Boija, A; Decker, TM; Rimel, JK; Fant, CB; Lee, TI; Cisse, II; Sharp, PA; Taatjes, DJ; Young, RA. RNA polymerase II phosphorylation regulates a switch between transcriptional and splicing condensates. Nature 2019, 572: 543 – 548.
Boija, A; Klein, IA; Sabari, BR; Dall'Agnese, A; Coffey, EL; Zamudio, AV; Li, CH; Shrinivas, K; Manteiga, J; Hannett, NM; Abraham, BJ; Schuijers, J; Afeyan, L; Guo, YE; Rimel, JK; Fant, CB; Lee, TI; Taatjes, DJ; Young, RA. Transcription factors activate genes through the phase separation capacity of their activation domains. Cell 2018, 175: 1842-1855.
Goodrich, JA; Taatjes, DJ. Gene regulation: a new phase in transcription. Nature 2018, 558: 197 - 198.

