Regulation of transcription and cell signaling by transcription-associated kinases
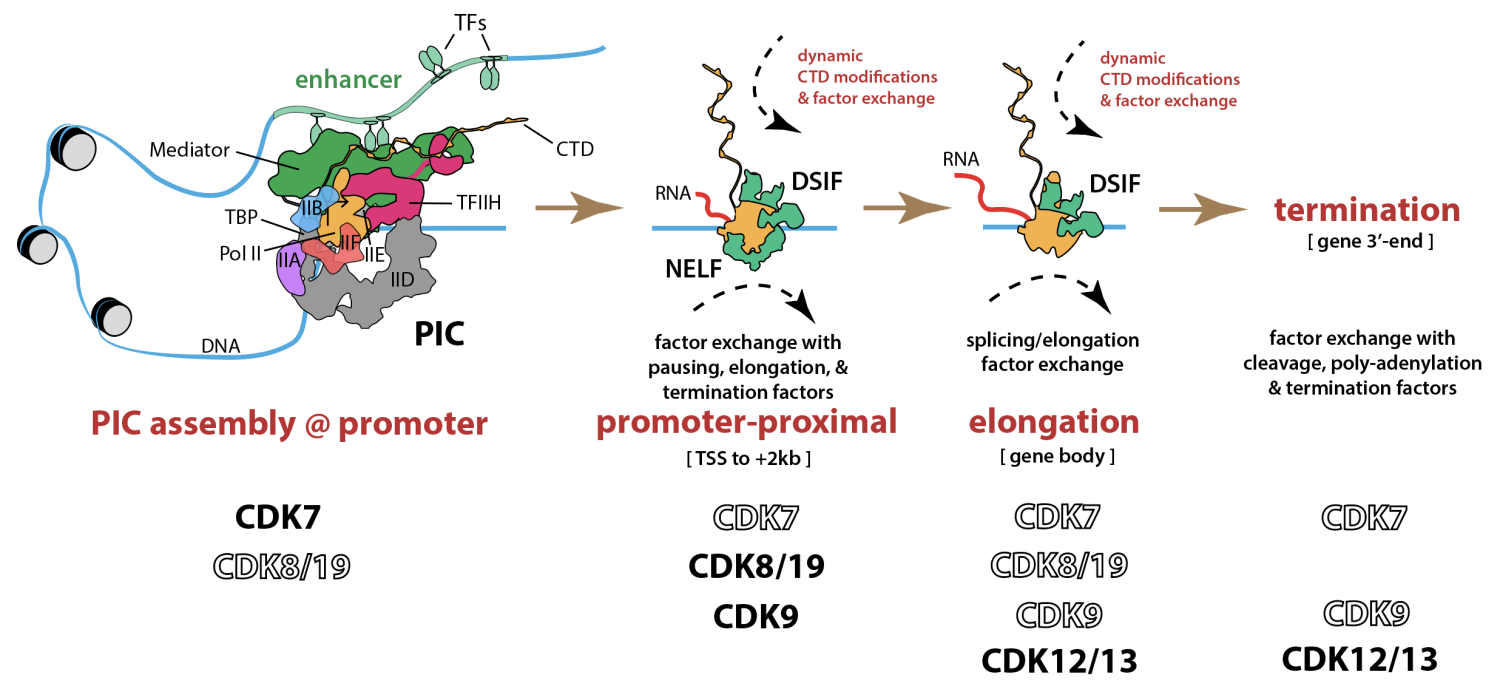
The transcription-associated kinases CDK7, CDK8/19, and CDK9 are essential for development and normal physiological function, and altered function of each kinase is broadly implicated in myriad human diseases. Every stage of RNAPII transcription (initiation, pause release, elongation, termination) and every stage of mRNA biogenesis (capping, splicing, cleavage, poly-adenylation, nuclear export) is regulated in part by transcription-associated kinases. Furthermore, kinases help control cellular responses to physiological cues (e.g. hormones or cytokines) through signaling cascades. Using a combination of biochemistry, chemical biology, chemical genetics, cell biology, transcriptomics, proteomics, and metabolomics, our lab is studying how the kinases CDK7, CDK8, and CDK9 coordinately control gene expression and cell signaling cascades in specific developmental or disease-associated contexts. The scheme below highlights how select kinases govern major regulatory stages of gene expression (adapted from Clopper & Taatjes Curr Opin Chem Biol 2022 102186 and Nagel & Taatjes Mol Cell 2025 1907). Note CDK19 is a vertebrate-specific paralog of CDK8.
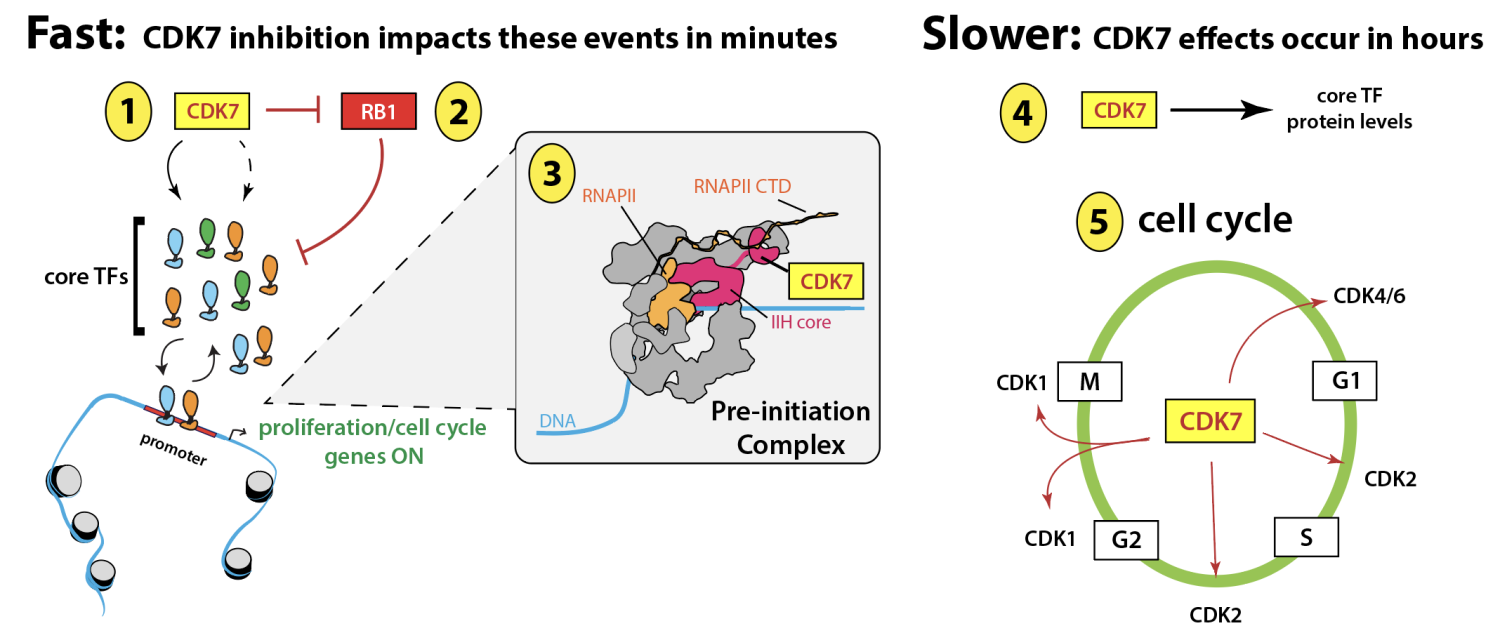
Sequence-specific, DNA-binding TFs coordinate all physiological processes by being responsive to signaling cascades and by directing the RNAPII machinery to specific genomic sites to establish cell- and context-specific gene expression patterns. Working with the Dowell lab (UC-Boulder), we recently discovered that a core TF network (representing n = 78 TFs) is "always on" in proliferating cells (about 80 different cell lines evaluated, representing 27 distinct tissue types; see Jones et al. Genome Biol 2025 92). This core network includes TFs from the ATF, E2F, ETS, KLF, and SP1 TF families. Remarkably, we separately discovered that CDK7 kinase activity is required to maintain core TF activity; CDK7 inhibition rapidly (within 30min) and uniformly shuts down core TF function, selectively blocking expression of their target genes, leading to cell cycle and growth arrest.
Since the 1990s, a persistent and confounding question related to CDK7 function has been whether its impact on cell cycle CDKs and RNAPII transcription are coordinated in any way. Through its kinase-dependent regulation of a core TF network, we identified a means by which CDK7 coordinates its functions as a cell cycle CDK-activating kinase with its control of RNAPII transcription (Jones et al. Sci Adv 2025 eadr9660). The core TFs controlled by CDK7 drive cell cycle and proliferative gene expression programs, which are functionally linked to cell cycle CDKs that are also governed by CDK7. In this way, CDK7 can at once coordinate proliferative gene expression programs with its regulation of cell cycle CDKs (see schematic below).
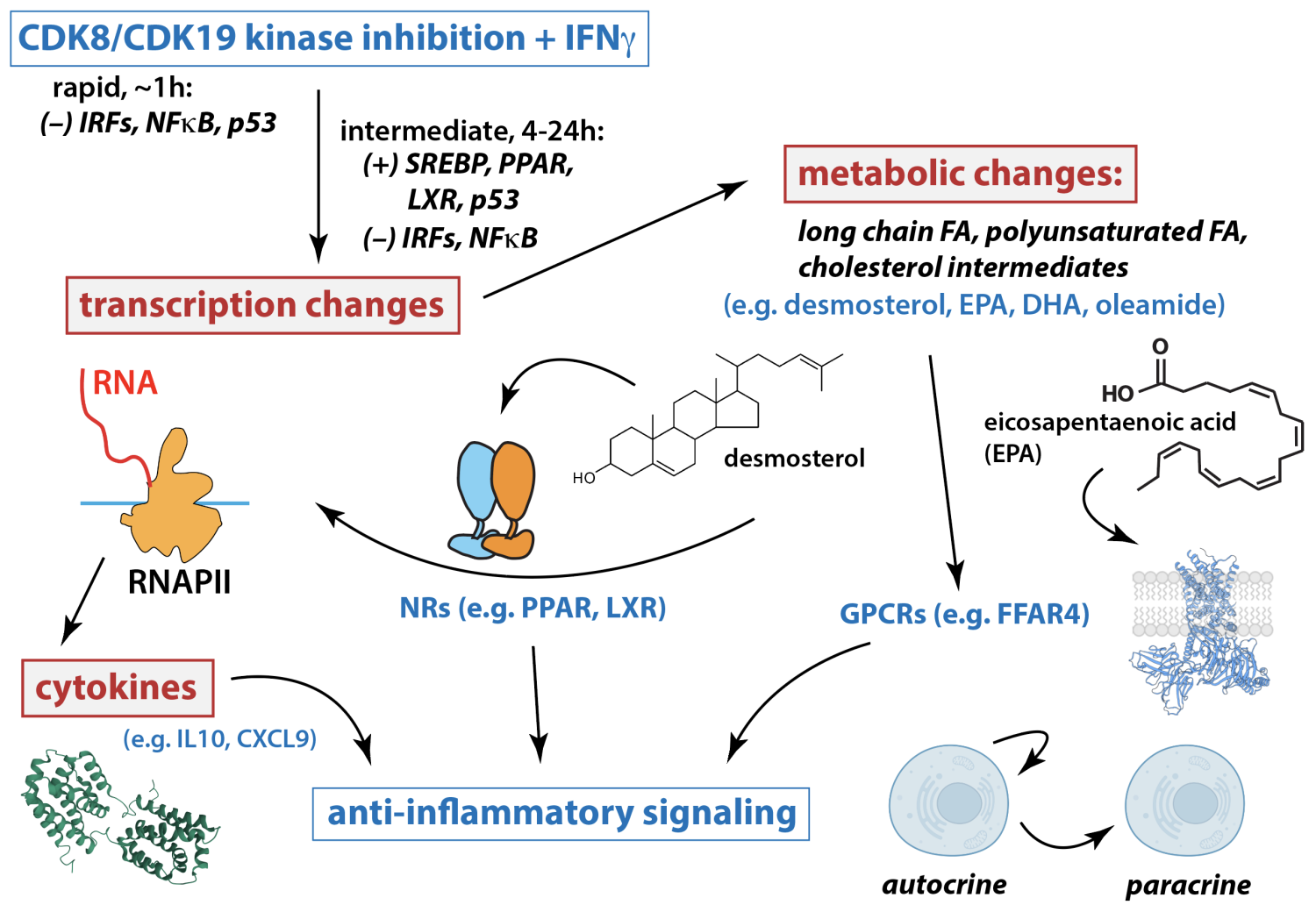
We also recently established a central role for the Mediator-associated kinases CDK8 and CDK19 in hyperactive interferon signaling in Down syndrome individuals. Moreover, this project revealed that CDK8/CDK19 regulate RNAPII splicing and we discovered fundamental metabolic functions for CDK8 and CDK19, revealing physiological roles beyond RNAPII transcription (Cozzolino et al. eLife 2025 RP100197). Hyperactive interferon signaling is a hallmark of Down syndrome, a condition caused by trisomy 21 (T21); in addition, chronic inflammation is linked to many distinct human pathologies. By demonstrating that Mediator kinase inhibition antagonizes pro-inflammatory interferon signaling in Down syndrome (see scheme below), we uncovered potential new therapeutic strategies to mitigate co-morbidities associated with Down syndrome or other chronic inflammatory conditions.
Recent publications related to this topic:
Luyties, O; Sanford, L; Rodino, J; Nagel, M; Jones, T; Rimel, JK; Ebmeier, CC; Palacio, M; Shelby, GS; Cozzolino, K; Brennan, F; Hartzog, A; Saucedo, MB; Watts, LP; Spencer, S; Kugel, JF; Dowell, RD; Taatjes DJ. Multi-omics and biochemical reconstitution reveal CDK7-dependent mechanisms controlling RNA polymerase II function at gene 5'- and 3'-ends. Cell Rep 2025, 44: 115904.
Jones, T; Feng, J; Luyties, O; Cozzolino, K; Sanford, L; Rimel, JK; Ebmeier, CC; Shelby, GS; Watts, LP; Rodino, J; Rajagopal, N; Hu, S; Brennan, F; Maas, ZL; Alnemy, S; Richter, WF; Koh, AF; Cronin, NB; Madduri, A; Das, J; Cooper, E; Hamman, KB; Carulli, JP; Allen, MA; Spencer, S; Kotecha, A; Marineau, J; Greber, BJ; Dowell, RD; Taatjes DJ. TFIIH kinase CDK7 drives cell proliferation through a common core transcription factor network. Sci Adv 2025, 11: eadr9660.
Cozzolino, K; Sanford, L; Hunter, S; Molison, K; Erickson, B; Courvan, MCS; Jones, T; Ajit, D; Galbraith, MG; Espinosa, JM; Bentley, DL; Allen, MA; Dowell, RD; Taatjes, DJ. Mediator kinase inhibition suppresses hyperactive interferon signaling in Down syndrome. eLife 2025, 13: RP100197.
Nussbaum, DP; Martz, CA; Waters, AM; Barrera, A; Liu, A; Rutter, JC; Cerda-Smith CG; Stewart, AE; Wu, C; Cakir, M; Levandowski, CB; Kantrowitz, DE; McCall, SJ; Pierobon, M; Petricoin, EF; Smith, JJ; Reddy, TE; Der, C; Taatjes, DJ; Wood, KC. Mediator kinase inhibition impedes transcriptional plasticity and prevents resistance to ERK/MAPK-targeted therapy in KRAS-mutant cancers. NPJ Precision Oncol 2024, 8: 124.
Johnson, JL; Yaron, TM; Huntsman, EM; Kerelsky, A; Song, J; Regev, A; Lin, T-Y; Liberatore, K; Cizin, DM; Cohen, BM; Vasan, N; Ma, Y; Krismer, K; Torres Robles, J; van de Kooij, B; van Klimmeren, AE; Andree-Busch, N; Kaufer, N; Dorovkov, MV; Ryazanov, AG; Takagi, Y; Kastenhuber, ER; Goncalves, MD; Hopkins, BD; Elemento, O; Taatjes, DJ; Maucuer, A; Yamashita, A; Degterev, A; Linding, R; Blenis, J; Hornbeck, PV; Turk, BE; Yaffe, MB; Cantley, LC. A global atlas of substrate specificities for the human serine/threonine kinome. Nature 2023, 613: 759 - 766.
Clopper, KC; Taatjes, DJ. Chemical inhibitors of transcription-associated kinases. Curr Opin Chem Biol 2022, 70: 102186.
Richter, WF; Nayak, S; Iwasa, J; Taatjes, DJ. The Mediator complex as a master regulator of transcription by RNA polymerase II. Nat Rev Mol Cell Biol 2022, 23: 732 - 749.
Luyties, O; Taatjes, DJ. The Mediator kinase module: an interface between cell signaling and transcription. Trends Biochem Sci 2022, 47: 314 - 327.
Rimel, JK; Poss, ZC; Erickson, B; Maas, ZL; Ebmeier, CC; Johnson, JL; Decker, T-M; Yaron, TM; Bradley, MJ; Hamman, KB; Hu, S; Malojcic, G; Marineau, JJ; White, PW; Brault, M.; Tao, L.; DeRoy, P; Clavette, C; Nayak, S; Damon, LJ; Kaltheuner, IH; Bunch, H; Cantley, LC; Geyer, M; Iwasa, J; Dowell, RD; Bentley, DL; Old WM; Taatjes, DJ. Selective inhibition of CDK7 reveals high-confidence targets and novel mechanisms for TFIIH function in transcription. Genes Dev 2020, 34: 1452 – 1473.
Steinparzer, I; Sedlyarov, V; Rubin, JD; Eislmayr, K; Galbraith MD; Levandowski, CB; Vcelkova, T; Sneezum, L; Wascher, F; Amman, F; Kleinova, R; Bender, H; Andrysik, Z; Espinosa, JM; Superti-Furga, G; Dowell, RD; Taatjes, DJ; Kovarik, P. Transcriptional responses to IFNg require Mediator kinase-dependent pause release and mechanistically distinct CDK8 and CDK19 functions. Mol Cell 2019, 76: 485 – 499.
Back to Research

