A crucial component of our work also involves collaborating with experimentalists to theoretically study challenging systems, such as metal-organic frameworks, covalent organic frameworks, atomic layer etching, atmospheric chemistry, strongly correlated condense matter systems and light-matter interaction in quantum cavities. A few examples are listed below.
Effective low energy Hamiltonian of Cuprates
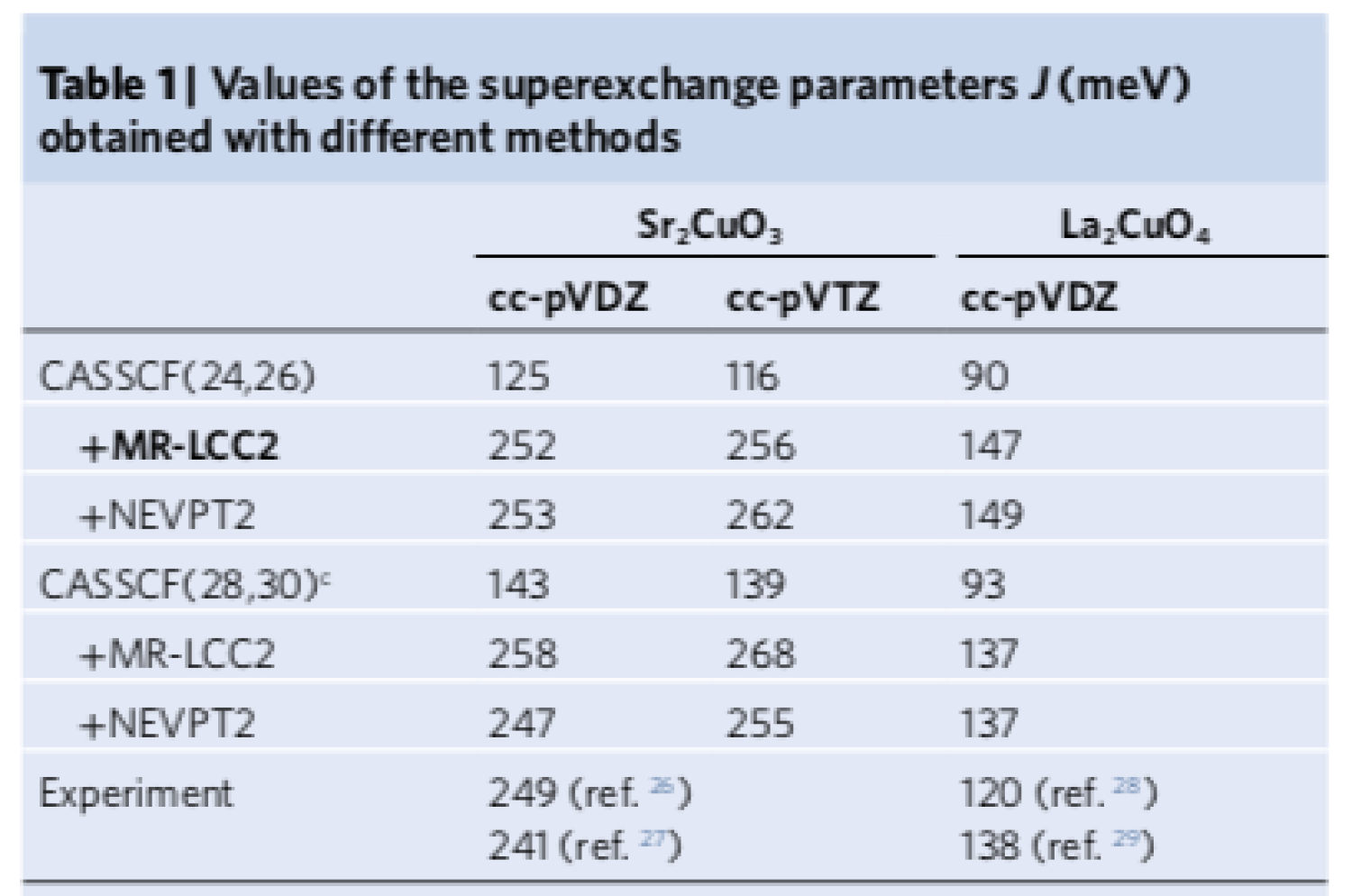
The electronic properties of cuprates are also known to be highly sensitive to the presence, distance and displacement of apical oxygens perpendicular to the CuO
2 planes. We performed ab initio quantum chemistry calculations using the multireference methods developed in our group to obtain the nearest-neighbour superexchange antiferromagnetic (AF) coupling
J of two cuprates, Sr
2CuO
3 and La
2CuO
4. The former lacks apical oxygens, whilst the latter contain two apical oxygens per CuO
2 unit completing a distorted octahedral environment around each Cu atom. Good agreement is obtained with experimental estimates for both systems. Analysis of the correlated wavefunctions together with extended superexchange models shows that there is an important synergetic effect of the Coulomb interaction and the O–Cu hopping, namely a correlated breathing-enhanced hopping mechanism. This is a new ingredient in superexchange models. Suppression of this mechanism leads to drastic reduction in the AF coupling, indicating that it is of primary importance in generating the strong interactions. We also find that
J increases substantially as the distance between Cu and apical O is increased.
Atomic Layer Etching (ALE)
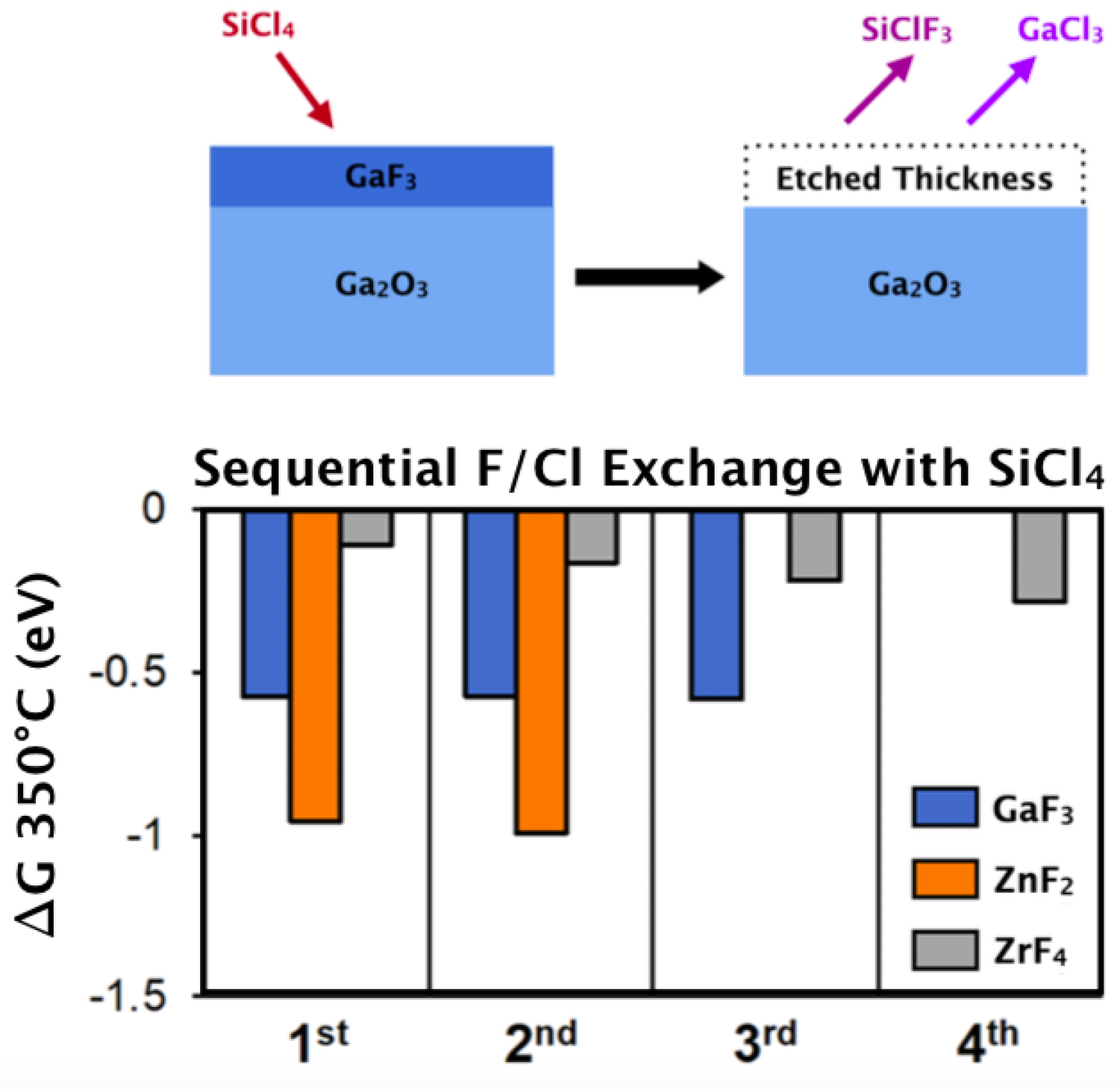
Thermal atomic layer etching (ALE) is a self-limiting process in which reactions liberate atomically thin layers of surface material. Several reaction mechanisms have been observed for successful ALE including ligand exchange, precursor addition, and ligand substitution hydrogen transfer. The viability of these reactions can be studied quantitatively via simple thermochemical methods. Translational, vibrational, and rotational degrees of freedom are calculated from ab initio values. These degrees of freedom, in conjunction with statistical mechanics, give a Gibbs free energy for the proposed reaction at the temperature of interest. Through this method we have been able to clarify mass spec observations, validate proposed novel mechanisms, and justify observed selective etching behaviors.
Atmospheric Rates with TST
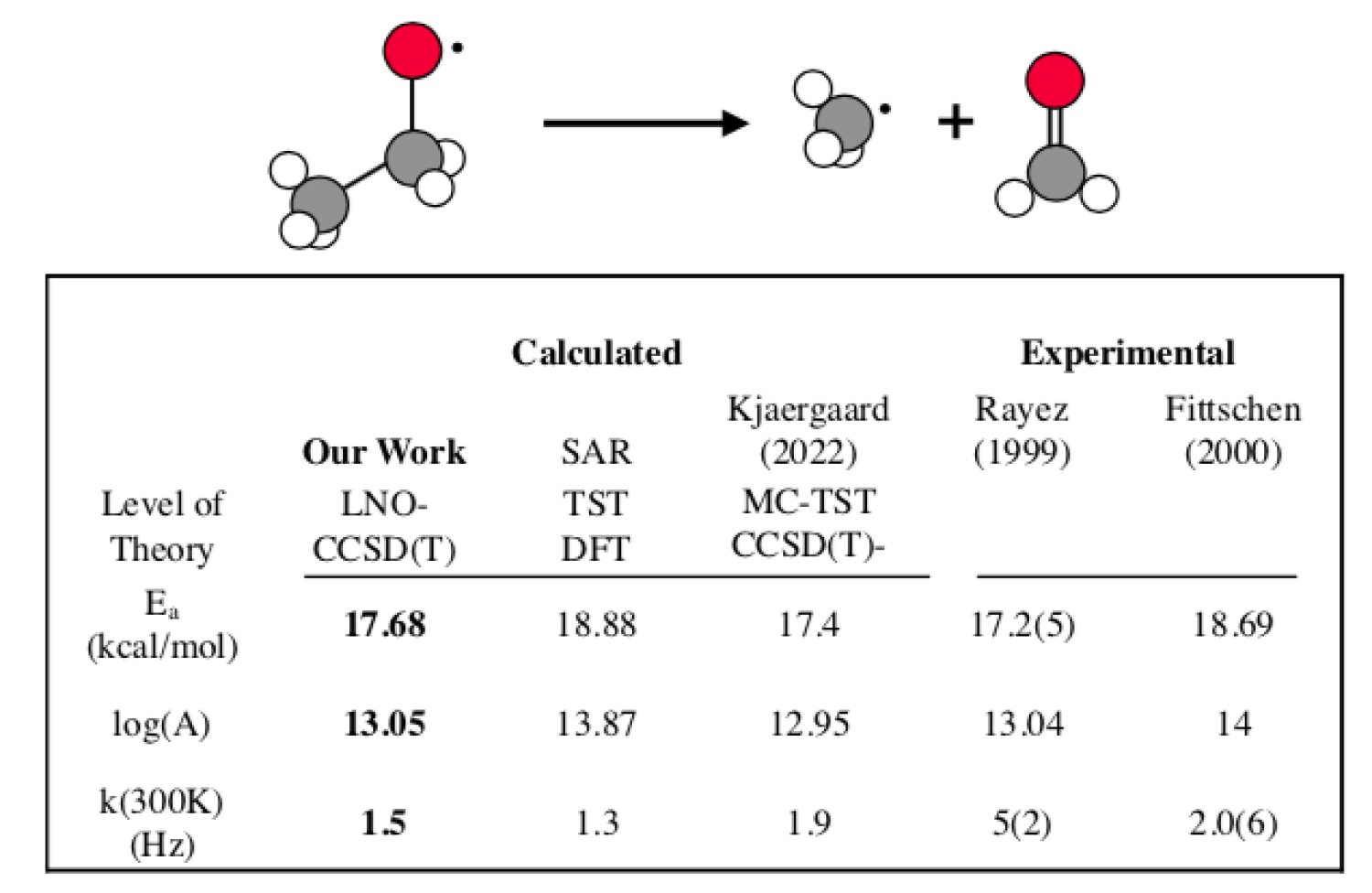
Alkoxy radicals are an important class of volatile organic compounds (VOCs) which react readily in the troposphere. The radical species can decompose through carbon-carbon bond scission, or stabilize through autoisomerization by liberating a hydrogen from their hydrocarbon chain. Autoisomerization proceeds through a 6-member transition state ring and is only relevant for sufficiently large alkoxy radicals. The result of these reactions has heavy implications for climate science and human health, but understanding these branching pathways can be complicated due to experimental limitations. Using transition state theory (TST) we are able to discuss these pathways in isolation or compare branching ratios. For small species we have found excellent agreement with experiment at the CCSD(T)/CBS level of theory and continue work on larger species.
Tetracene dimers for triplet pair production
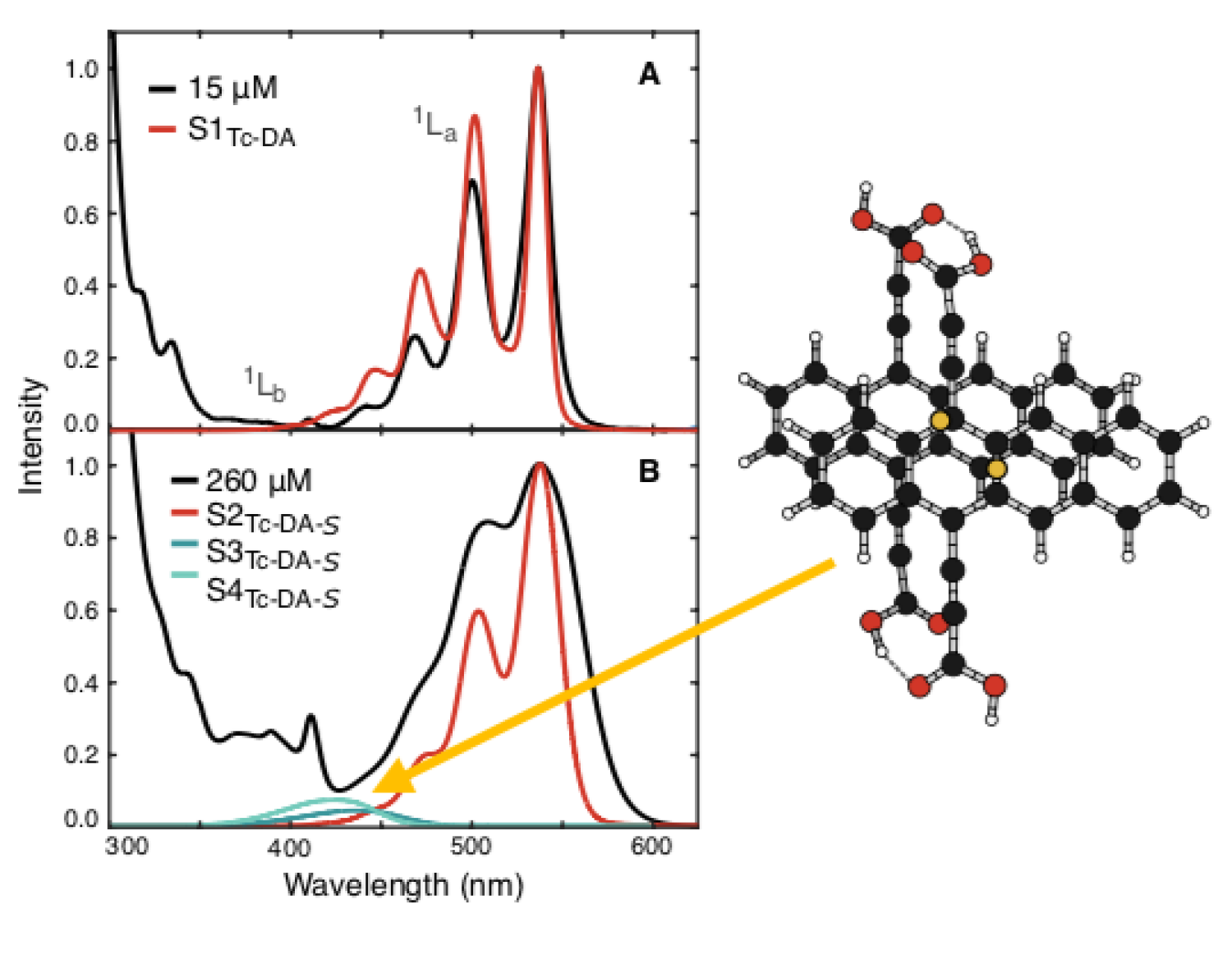
Some organic chromophores, when coupled, can produce a pair of coupled triplets from a photoexcited singlet state through singlet fission. It is believed that energy or charge transfer states play a role in singlet fission and these states are of interest to biomedical, solar-energy harvesting, and light-generating technologies. Using DFT, we predicted ground state geometries for new tetracene diacid dimers. Their vibronic spectra were calculated using ground state vibrational energies and excited state electronic energies from TD-DFT. Our work identified signals in their UV-VIS spectrum from dimer states, of charge transfer character, that indicate dimer formation.