Biochemist wins $750k for novel cancer research
CU Boulder professor recognized for her research on cancer drug resistance
Sabrina L. Spencer, assistant professor of biochemistry and member of the BioFrontiers Institute at the University of Colorado Boulder, is one of five new winners of a 2021 Damon Runyon-Rachleff Innovation Award, the Damon Runyon Cancer Research Foundation announced recently. She is also one of seven new winners of the 2021 Emerging Leader Award from the Mark Foundation for Cancer Research.

At the top of the page: 3D illustration of proteins with T cells (National Institutes of Health National Cancer Institute). Above: Sabrina L. Spencer.
The two New York City-based foundations are co-funding the work to study causes and consequences of rapid cancer cell adaptation to inhibitors of a key cell proliferation pathway called the MAPK pathway.
Both foundations aim to fund promising early career investigators whose innovative work addresses unmet needs in cancer research.
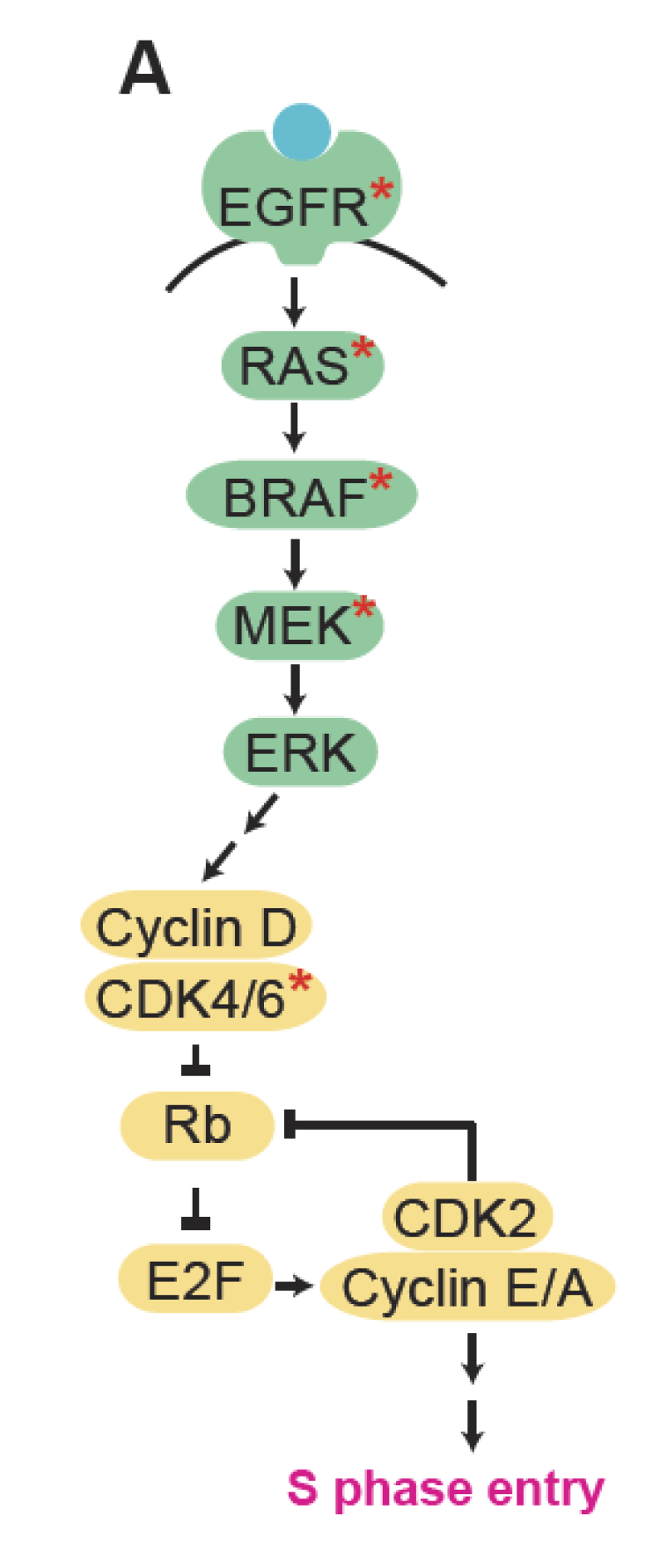
Schematic of MAPK signaling (green) driving cell-cycle entry (yellow). Red stars mark targets to be drugged in the proposed work.
Specifically, the Damon Runyon-Rachleff Innovation Award funds cancer research by “exceptionally creative thinkers” who have “high-risk/high-reward” ideas but who lack sufficient preliminary data to obtain traditional funding.
Damon Runyon-Rachleff Innovation Award winners are selected through a competitive process by a scientific committee of leading cancer researchers who are innovators themselves.
“Only those scientists with a clear vision and passion for curing cancer are selected to receive the prestigious award,” the foundation states.
The Mark Foundation Emerging Leader Award funds scientists who “take on innovative, high-risk/high-reward projects that have significant potential to improve outcomes for cancer patients” and aims to “encourage the next generation of oncology superstars.”
As an early career scientist whose work has the potential to “significantly impact the prevention, diagnosis and treatment of cancer,” Spencer will receive $750,000 over three years, co-funded by the two foundations.
Research in Spencer’s lab is broadly focused on understanding how signaling events control cell fate. Studying these processes in single cells reveals remarkable cell-to-cell variability in response to stimuli, even among genetically identical cells in a uniform environment.
“We seek to understand the causes and consequences of this heterogeneity in the cellular response to stimuli,” she states. “Our long-term goal is to understand the normal functioning of signaling pathways that control proliferation, to understand how these signals go awry in cancer, and eventually to alter the fate of individual cells.”
In the newly funded work, Spencer turns her efforts toward understanding the inception of cancer drug resistance. Until recently, basic research in oncology has primarily focused on mutations that drive cancer drug resistance, Spencer states in an abstract of her proposal. While the contribution of mutations to cancer drug resistance is undeniable, how cells adapt to drugs on short time scales, long before genetic mutations arise, is very poorly understood.
Spencer will use multi-day time-lapse microscopy to study how individual cancer cells adapt to drugs targeting the MAPK pathway during the first few days of drug treatment. She will identify the molecular events that allow individual rogue cells to escape from drug action.
Preliminary evidence from the Spencer lab suggests that these drug “escapees” incur DNA damage while laboring through the cell cycle in the presence of MAPK inhibitors, potentially enabling the future development of permanent (genetic) drug resistance in this subpopulation.
This is important because these early drug “escapees” could represent a seed population driving drug resistance, and, if understood, could be therapeutically blocked to reduce tumor relapse, her abstract notes.
“I am very honored to receive these votes of confidence in my ideas from both the Mark Foundation for Cancer Research and the Damon Runyon Cancer Research Foundation,” Spencer said.
“If our hypotheses turn out to be correct, the results could change our understanding of the origins of drug resistance to put more focus on the early response of cells to drugs. This would enable identification of combination therapies that could prevent cells from rapidly adapting to and evading MAPK drug treatment, perhaps by blocking stress-response pathways,” she said, adding:
“Because so many different kinds of cancers are treated with various drugs that target MAPK-associated signaling, this work could have broad implications across a spectrum of cancer types.”
Spencer earned her PhD in computational and systems biology from the Massachusetts Institute of Technology in 2009 and completed a postdoctoral fellowship at Stanford University before joining the CU Boulder faculty in 2014.
In 2016, she was named a Searle Scholar, a Kimmel Scholar, a Beckman Young Investigator and a Boettcher Early Career Investigator. In 2017, she was named a Pew-Stewart Scholar, and in 2018, an American Cancer Society Research Scholar and a National Institutes of Health Director’s New Innovator.

