Effect of Chelation on Iron–Chromium Redox Flow Batteries
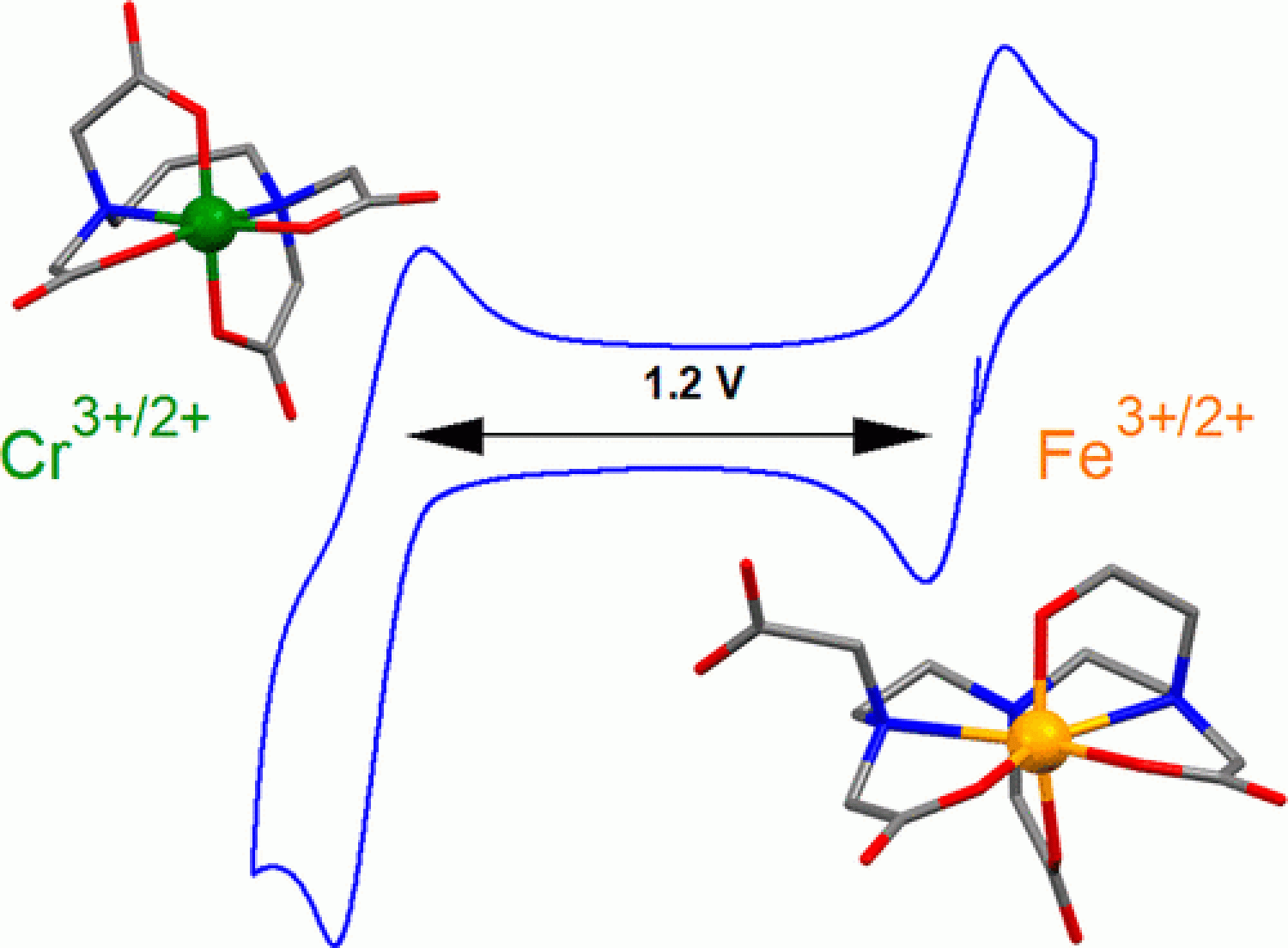
The iron–chromium (FeCr) redox flow battery (RFB) was among the first flow batteries to be investigated because of the low cost of the electrolyte and the 1.2 V cell potential. We report the effects of chelation on the solubility and electrochemical properties of the Fe3+/2+ redox couple. An Fe electrolyte utilizing diethylenetriaminepentaacetic acid (DTPA) exhibits efficient and high-performance flow battery cycling at pH 9 versus a Cr-chelate complex utilizing 1,3-diaminopropanetetraacetic acid (PDTA). The FeDTPA electrolyte can be cycled at concentrations up to 1.35 M, equating to a storage capacity of 36.2 Ah L–1, with near-quantitative efficiency. When paired with a CrPDTA electrolyte, the equilibrium cell potential of the all-chelated FeCr RBF is 1.2 V with a maximum discharge power of 216 mW cm–2. Key aspects of the coordination chemistry of FeDTPA are compared with CrPDTA and highlight the importance of molecular-level understanding for driving flow battery system performance.