Chelated Chromium Electrolyte Enabling High-Voltage Aqueous Flow Batteries
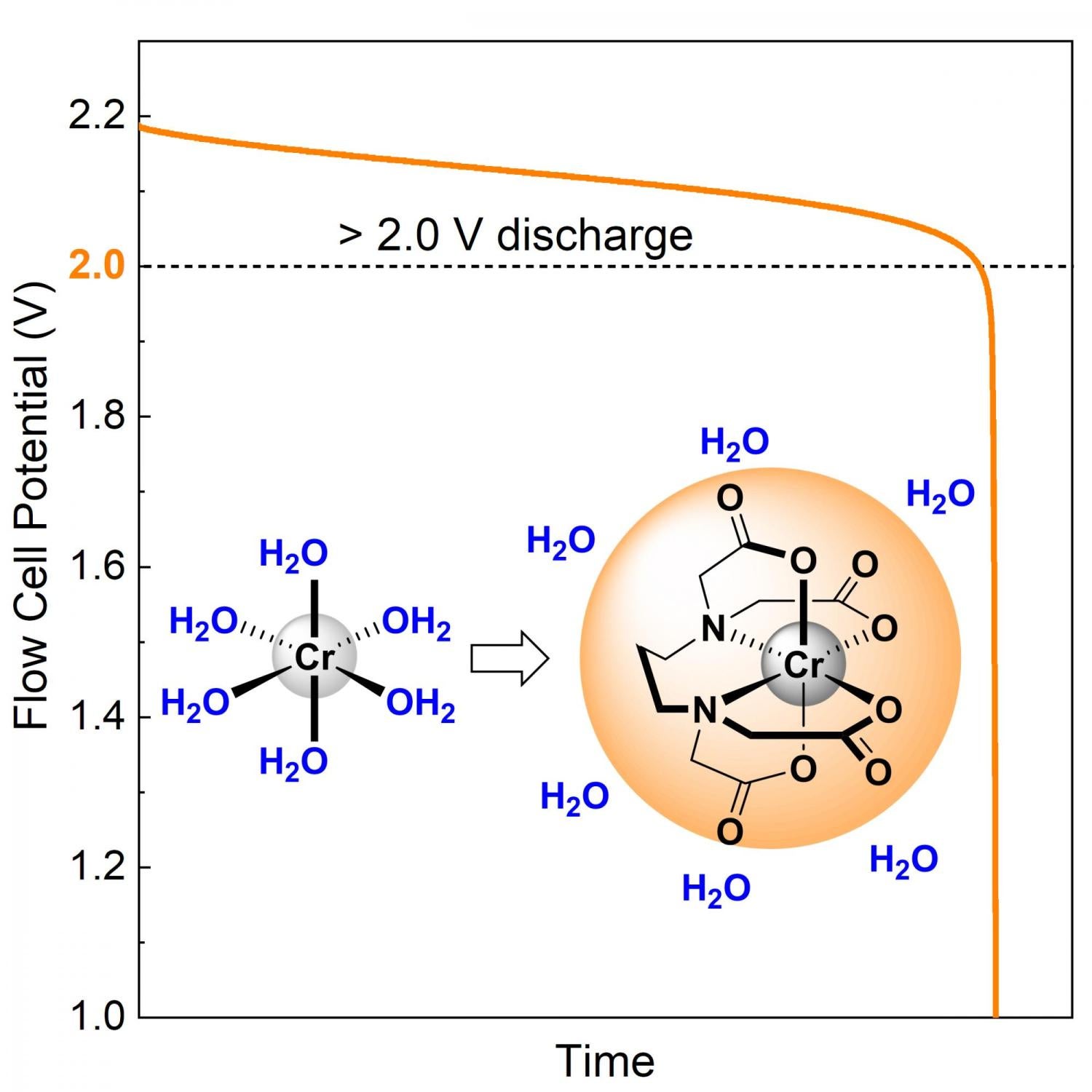
Widespread adoption of renewable energy is limited by the lack of low-cost long-duration energy storage. Redox flow batteries are an attractive option to provide this type of storage because their power and energy components can be scaled independently; however, systems commercialized to date have failed to realize this low-cost potential, primarily because of the cost and performance of the battery chemistry. Here, we resolve these challenges with a negative electrolyte comprised of earth-abundant chromium and an inexpensive chelating agent which operates at a more reducing potential than zinc while inhibiting hydrogen production. Utilizing this electrolyte, we report two of the highest voltage aqueous flow batteries, which have stably operated at room temperature and near neutral pH with high efficiency and high power density. These results demonstrate the ability of chelated metal complexes to prevent water coordination and establish a general method for inhibiting water splitting reactions at highly negative potentials.