Alex Khang
- Postdoctoral Researcher
- BIOFRONTIERS INSTITUTE
Curriculum Vitae
Research Interests
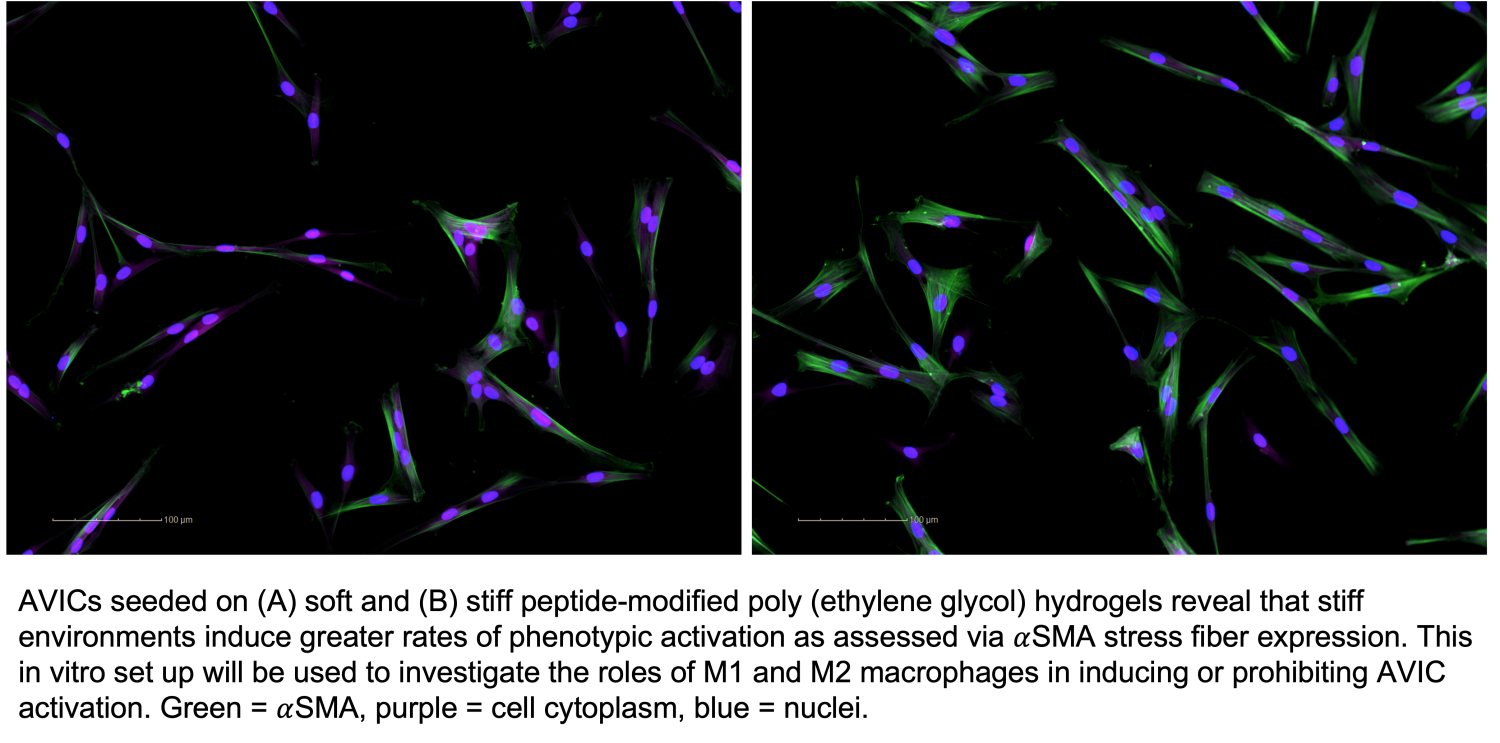
Aortic valve stenosis (AVS) occurs when the aortic valve (AV) stiffens, causing it to narrow and be unable to open properly. Intriguingly, the mechanisms that potentiate AVS differ based on biological sex. Clinical studies have shown that among patients with the same AVS severity, male AVs tend to present with higher levels of calcification while female AVs are more fibrotic overall. AVS progression, to a large extent, is driven by the underlying aortic valve interstitial cell (AVIC) population, whose function can be influenced by infiltrating macrophages. AVICs maintain a quiescent phenotype during physiological periods and can take on an activated myofibroblast phenotype in response to altered biomechanical or biochemical cues. Common hallmarks of AVIC activation include increases in collagen production, extracellular matrix (ECM) remodeling, and contractility brought about through expression of alpha-smooth muscle actin (SMA) stress fibers. As it stands, little is known regarding how interactions between macrophages and AVICs lead to sexual dimorphisms in AVS progression. My research entails using tissue-emulating, peptide-modified hydrogels as a culture platform to elucidate the roles of pro-inflammatory (M1) macrophages and reparative (M2) macrophages in inducing a pro-calcific or pro-fibrotic AVIC response. These highly tunable hydrogel platforms allow for precise control of the mechanical and biochemical properties of the cellular micro-environment and critically allow for realistic co-culturing of macrophages and AVICs.