Active Matter
Cells are known to change their mechanical and physical properties upon maturation and disease. The ability to easily and quickly detect these changes over time is a key to early cancer prognosis or increasing the outcome of in-vitro fertilization procedures. Our team develops computational models of the cell cytoskeleton and its surrounding cortical membrane with the aim of detecting its biological state through its “mechanical” signature. The characterization and detection steps are pursued via two approaches:
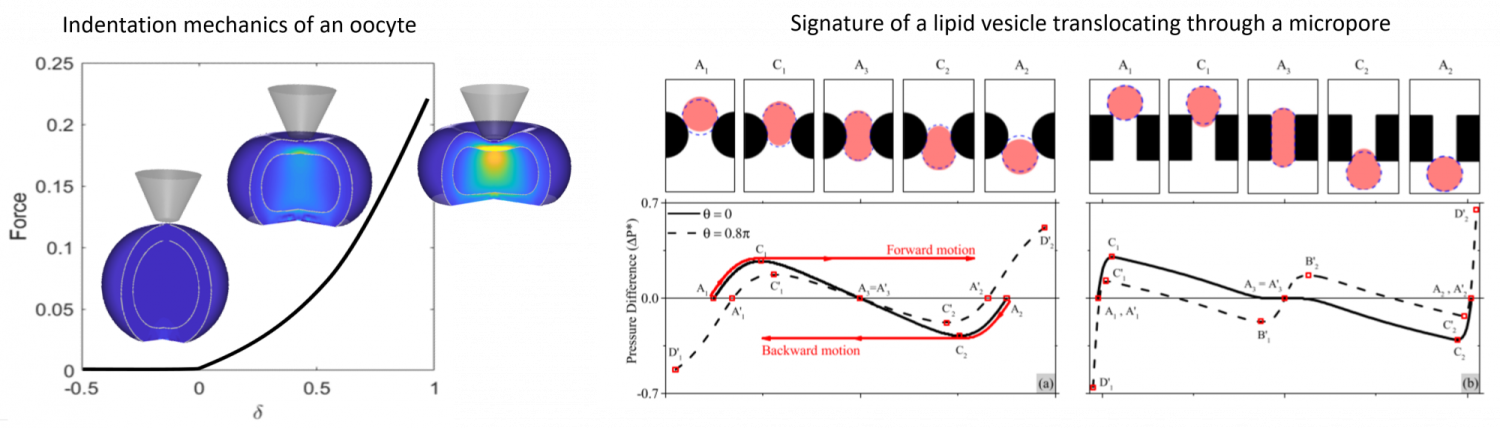
- Assessment of cell mechanics via indentation and pipette aspiration. In collaboration with the metrology group at FEMTO (University of Franche Comte, France), we study the physical changes of human oocytes during maturation. By linking the probability of an oocyte fertility to its mechanical properties (and structural changes), the model will be used to adequately screen and select optimal cells for in-vitro fecondation.
- Detection of abnormal cells (or virus) via their mechanical signature. Abnormal cells (such as cancerous cells in the blood) usually exhibits different mechanical properties from their healthy counterpart, but their detection is difficult since screening involve a very large number of cells. High throughput screening can however be acheived by flowing cells in small pores (or microfluidic devices) by pressure-driven flow or electrophoresis. As a cell passes through a pore, it generates a change in flow pressure (or electrical signal) that depends on pore shape, cell size and properties which can be detected in real time. We use computational mechanics to link cell mechanics to its signature (see figure below). This work in is collaboration with the membrane science group of John Pellegrino (CU Boulder).