2005
 We examine the role of electronic polarizability in water on short (tens of femtoseconds), intermediate (hundreds of femtoseconds), and long (≈1 ps) time scales by comparing molecular dynamics results to experimental data for vibrational
We examine the role of electronic polarizability in water on short (tens of femtoseconds), intermediate (hundreds of femtoseconds), and long (≈1 ps) time scales by comparing molecular dynamics results to experimental data for vibrational Although it is widely accepted that the local structure of liquid water has tetrahedral arrangements of molecules ordered by hydrogen bonds, the mechanism by which water molecules switch hydrogen-bonded partners remains unclear. In this mechanism,
Although it is widely accepted that the local structure of liquid water has tetrahedral arrangements of molecules ordered by hydrogen bonds, the mechanism by which water molecules switch hydrogen-bonded partners remains unclear. In this mechanism,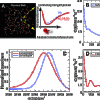 We present a microscopic description of the vibrational spectroscopy of the OH stretch of HOD in liquid D2O. Our model predicts that OH frequency correlations decay with a sharp and rapid (≈35 fs) decrease, followed by a beat at ≈125 fs from
We present a microscopic description of the vibrational spectroscopy of the OH stretch of HOD in liquid D2O. Our model predicts that OH frequency correlations decay with a sharp and rapid (≈35 fs) decrease, followed by a beat at ≈125 fs from

