Polarization-selective femtosecond Raman spectroscopy of low-frequency motions in hydrated protein films
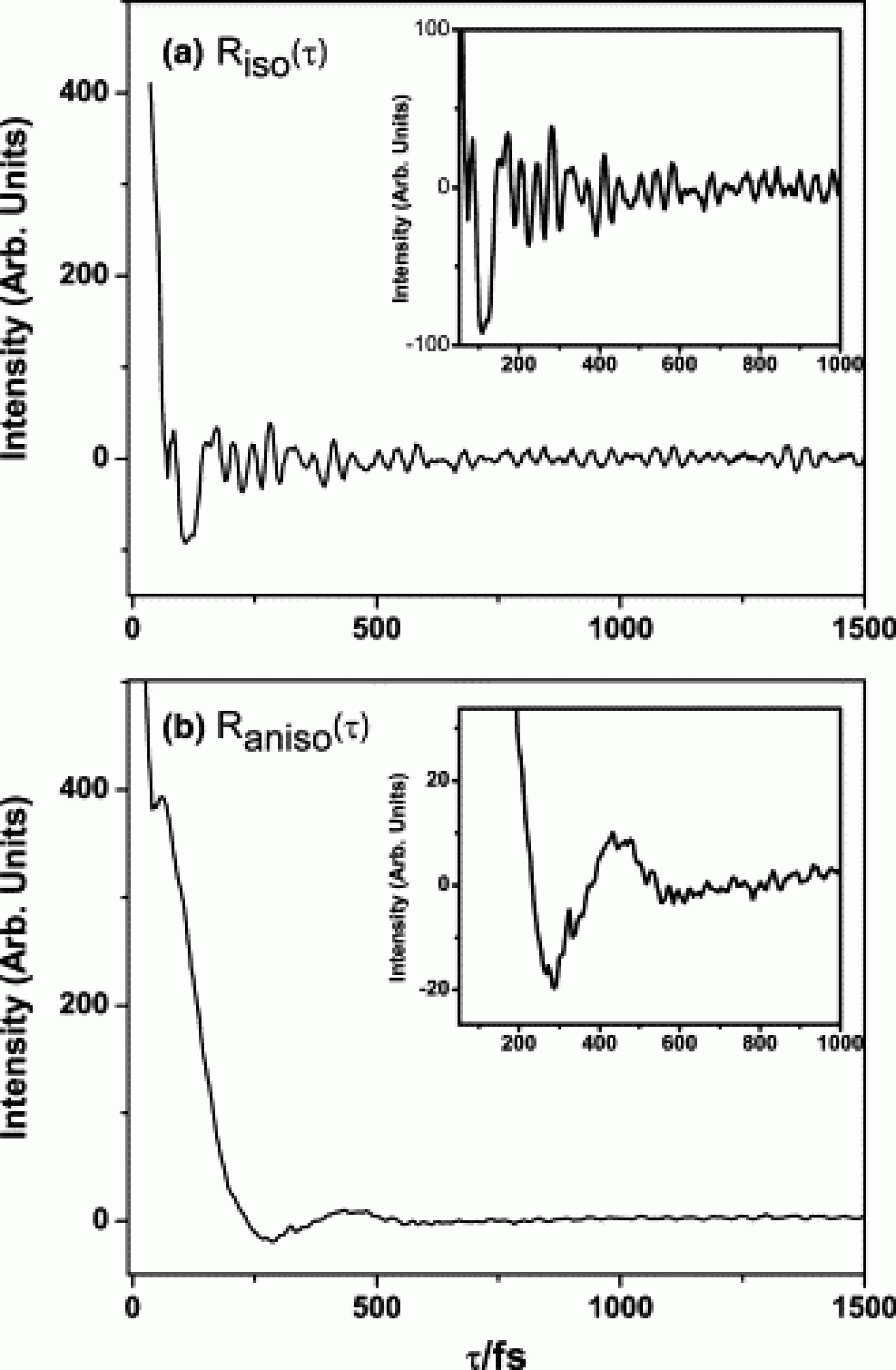
We investigated the vibrational dynamics of proteins in amorphous hydrated films of lysozyme and myoglobin using polarization-selective time-domain Raman spectroscopy. The anisotropic spectra for these proteins all have a broad peak due to librational motion of side chains at 90 cm−1 and a background that may arise from bound water. The isotropic spectrum of lysozyme is similar to that of myoglobin, and has peaks at 240 and 500 cm−1 that are likely due to secondary structure fluctuations. These results suggest that low-frequency deformations of the protein molecule may contribute to the solvation dynamics of proteins in aqueous solution.
Eaves, J. D., Fecko, C. J., Stevens, A. L., Peng, P. and Tokmakoff, A. Polarization-selective femtosecond Raman spectroscopy of low-frequency motions in hydrated protein films. Chem. Phys. Lett. 376, 20 (2003)

