Scientists move microscopic solar chemical factories out of water to unlock new transformations
Four RASEI Fellows work together to expand the potential applications of nanoparticle photocatalysts
We all understand the power of the sun. We feel it on a hot summer’s day, we see it harnessed in solar panels that power our homes and cities. Chemical photocatalysis develops approaches to shrink that ability to harness this energy down to a molecular scale, and uses this energy to power chemical reaction, to power the building of important organic molecules, the foundations of pharmaceuticals, materials, and clean fuels.
One of the technologies used to harness light on the molecular level are a class of particles called organic nanoparticles (oNPs). Think of them as tiny, solar-powered factories, expertly designed to capture light and use its energy to drive chemical reactions. The oNPs are made from readily available earth-abundant materials, offering a cheap, clean, and sustainable alternative to a range of more traditional chemical reactions, which can often rely on rare, expensive metals that are hard to get hold of and can produce significant waste.
However, the oNPs, for all their potential as chemical factories, do have one significant limitation, they can only be built and operated in water. This is a fundamental roadblock. While water is essential for life, it is often a very poor environment for performing chemical reactions and can be very detrimental for the complex and delicate sequence of chemical transformations required for producing valuable products. The full power of these nano-factories was, quite literally, stuck in the water.
To understand this challenge, imagine that you have designed the world’s most efficient and powerful engine. It is a true engineering breakthrough, but it comes with a major catch, it can only run while completely submerged in the ocean. This is fine if you want to get around in a submarine, or a boat, but you can’t put it into a car, or a plane, or a generator on land, where you need it most. The potential of this new innovation is trapped, unable to be used for countless valuable applications.
That is similar to the situation faced by the researchers, led by RASEI Fellows Stephen Barlow, Seth Marder, Obadiah Reid and Garry Rumbles. The oNPs were confined to water-based reaction media, in order to realize their full potential the team needed to find a way to move these delicate ‘nano-factories’ from water to the ‘dry-land’ of other chemical environments, known as non-aqueous solvents, without the oNPs collapsing or breaking down.
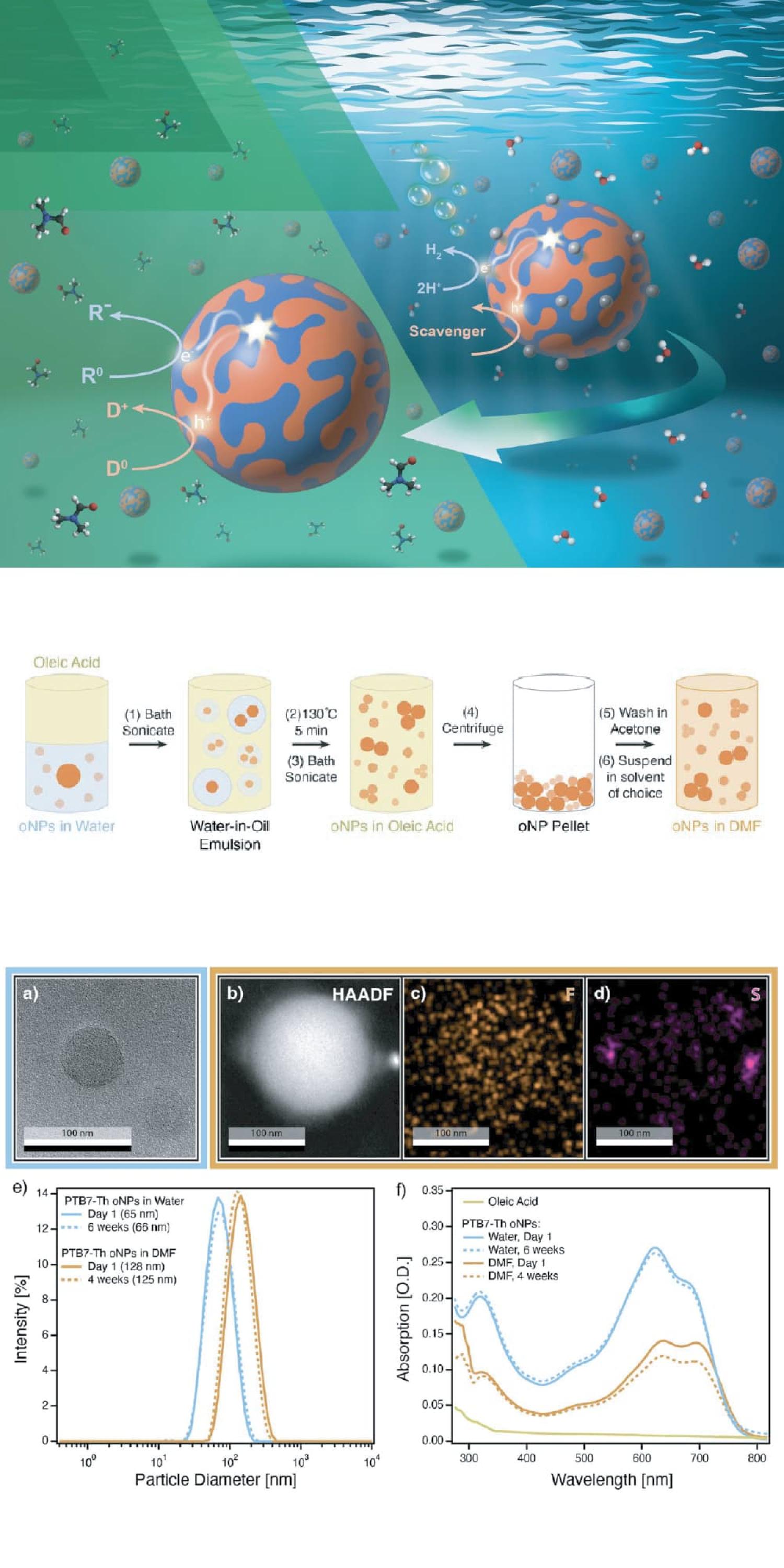
This study describes a solution that the team developed. They devised a multi-step process to gently coax the oNPs out of their native water environment and prepare them to operate in different chemical solvents.
The process they developed is analogous to making salad dressing in the kitchen. It begins by mixing the water containing the nanoparticles with an oily substance (in this study oleic acid) and shaking it. This creates an emulsion, where tiny droplets of water are suspended in the oil, much like a vinaigrette. During this process the oNPs leave the water and move into the oil, which wraps around them like a protective coating. Finally the water is gently remove, leaving the oNPs safely suspended in their new oily environment. The protective layer formed around them allows them to be seamlessly transferred to a range of new non-aqueous solvents, ready to get to work.
A key part of this study was demonstrating that the oNP ‘factories’ were still functional after their transfer to new solvent systems. Using a suite of tools the team were able to confirm that the transferred oNPs could still absorb light and perform the chemical reactions.
With these findings the potential of the oNPs can be explored and expanded. It reveals new opportunities, not only in putting together organic molecules, but also in the synthesis of clean fuels, such as hydrogen.