Shining new light on an old problem: Breaking down ‘Forever Chemicals’ and building the next generation of materials
Using a new catalyst and visible light, researchers have developed a chemical “scalpel” to degrade persistent pollutants and enable new, more precise chemical reactions.
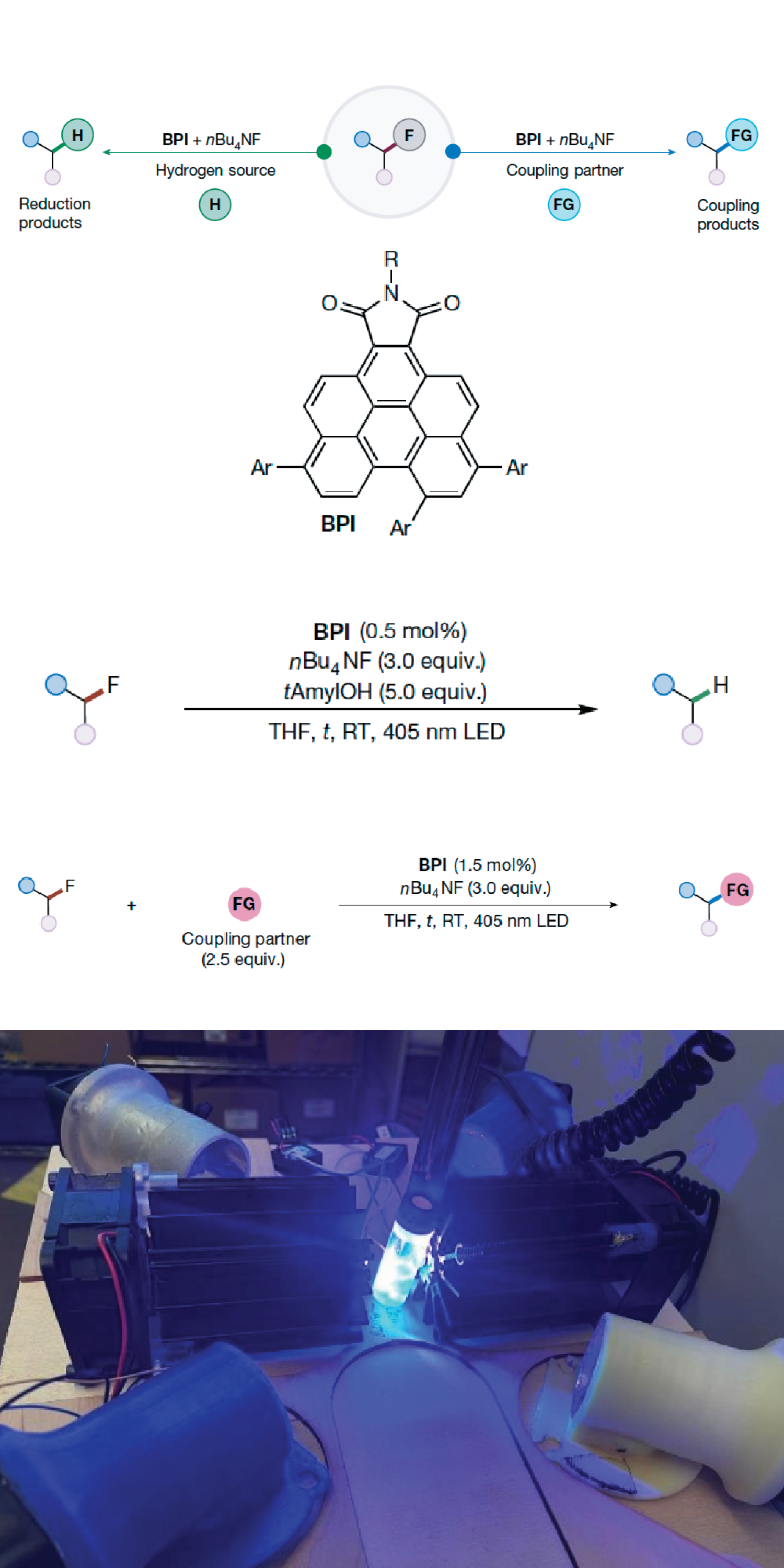
The global challenge of “forever chemicals” has made the headlines for years. The carbon-fluorine (C–F) bond is one of the strongest in all of chemistry. For decades the sheer strength of the C–F bond has been a blessing and a curse. This incredible strength is what makes “forever chemicals”, like PFAS, so stable and useful in everything from non-stick pans to waterproof clothing but is also the reason that they are nearly impossible to break down. This has been a major contributor to the growing plastic waste crisis, and a key reasons for these compounds to be now known as one of the hardest pollutants to remove from the environment. A recent collaborative study, including RASEI Fellow Niels Damrauer, has a new solution for this problem. Development of a new catalyst that acts like a chemical scalpel, using blue light to precisely sever this famously inert bond. This approach not only offers a new way to degrade these persistent pollutants but also opens the door to using what were previously considered unreactive fluorinated molecules as building blocks for new chemical transformations and products.
This study is an illustrative example of a modern, collaborative approach to chemical innovation, as part of the NSF funded Center for Sustainable Photoredox Catalysis (SuPRCat). The research team came at the problem from two different angles: computational modeling and hands-on experimentation. The computational chemists first used powerful simulations to design and predict the behavior of a new organic catalyst. This helped them understand exactly how the catalyst could use low-energy, visible blue light to act as our "chemical scalpel," targeting and breaking the C–F bonds without the need for intense heat.
With this knowledge, the experimental chemists then created the catalyst in the lab. They showed that it could precisely snip the C-F bonds in a variety of molecules, demonstrating it was capable of both degrading persistent pollutants like PFAS and building new chemical structures that were previously difficult to construct.
The success of this research with a simple, abundant energy source like visible light shows that chemical reactions don’t have to be energy intensive. This research describes the power of precise, light-driven chemistry. By designing a catalyst that can target and activate some of the toughest bonds in chemistry, this team has not only revealed a potential path forward for degrading PFAS, but also demonstrated a new tool for chemists to build molecules in a cleaner and more energy efficient way.