Resurrected microwave tool illuminates cutting-edge light-driven chemistry
A century old technique has been revitalized to provide valuable insights for catalyzing chemical reactions.
Using advanced computer-aided design and coding tools, a long-overlooked microwave analysis technique uncovered new insights into cutting-edge, light-driven chemistry. The technique revealed that a component of the catalyst often considered inconsequential is actually responsible for a four-fold change in chemistry.
Organic chemistry underlies many of today’s manufacturing processes from pharmaceuticals to plastics, but these processes can often generate hazardous byproducts. Catalysts are a critical tool in for transitioning organic chemistry to a greener, more sustainable future. By accelerating the rate of a chemical reaction without itself being consumed, a catalyst molecule more efficiently uses raw materials, eliminates waste, and can avoid the use of toxic and hazardous substances. New research from a team of scientists at the Princeton University and the Renewable and Sustainable Energy Institute (RASEI; a joint institute between the National Renewable Energy Laboratory (NREL) and University of Colorado Boulder (CU Boulder)) reveals key insights into how an important class of catalysts operate and offers a path to improving their performance.
Photoredox catalysts, that harness the energy from light (photo-) to transfer electrons to, or from, other molecules (what chemists refer to as reduction and oxidation respectively (hence ‘redox’)), takes the sustainability of catalysts to a higher level, by enabling reactions driven by light. Today, many researchers are seeking to understand how these photoredox catalysts systems work and to use this understanding to improve the efficiency of the redox reaction so it can be used at industrial scales. Many different variables can be modified to tune the reaction, such as the structure of the catalyst and the conditions of the reaction. A new discovery, published in Nature Chemistry in April 2022, revitalizes an long-overlooked analytical technique that has revealed the importance of a variable previously thought to be insignificant and uncover a new approach for improving these processes.
The breakthrough results from a collaboration between researchers from NREL and Princeton University. Light-driven catalysis has undergone a renaissance in recent years, and the Knowles group at Princeton have been one of the pioneers. Not only do these systems provide more sustainable alternatives to existing chemical transformations, but the gentler, more selective conditions under which they operate, (using light, instead of heat, or harsh chemicals), makes possible new and societally impactful chemistry.
However, to use these new reactions at manufacturing scale, a detailed understanding of how the reaction works is required. While organic chemists are fantastic at discovering and developing new reactions, they typically don’t have the tools to probe the details of a reaction. Enter the Rumbles-Reid group at RASEI—a team of physical inorganic chemists who specialize in investigating the “how” of reactions. By reviving an overlooked characterization technique, the RASEI team revealed unexpected details about how these catalysts work. This collaboration was made possible by an Energy Frontier Research Center (EFRC) funded by the U.S. Department of Energy, a collaborative project that brings together expertise from across a range of scientific disciplines. The Bioinspired Light-Escalated Chemistry (BioLEC) EFRC mission has a strong overlap with the RASEI sustainability goals. The team, led by Gary Rumbles and Obadiah Reid, used computational methods to modernize a technique that, despite being over 100 years old (it was first reported in 1915), has found little application in main-stream chemistry.
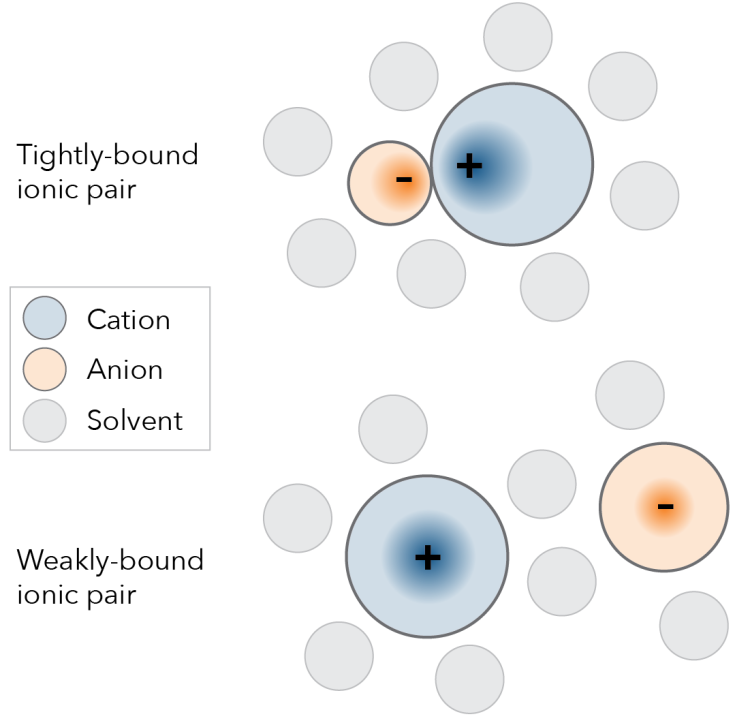
The photoredox catalyst exists as a pair of molecules, called an ionic pair, so named because each half carries an electronic charge. One half, that contains the metal iridium and is the molecule that interacts with light, is the cation (it has a positive (+) charge because it is ‘missing’ an electron), and the other half is called the anion (it has a negative (–) charge because it has an ‘extra’ electron). Consider the situation when you have two magnets, the opposite poles, or charges, attract, and in the solid form the anion (–) and cation (+) of the catalyst are tightly paired. When the ionic pair is placed in solution to perform the reaction it interacts with the molecules of the solvent, and the attraction between the pair is reduced. When you separate a pair of magnets there is still attraction, albeit weaker, and this is the same for the ionic pair. Common thinking is that during the reaction, while the iridium containing cation is being excited by the light energy, the anion is merely a bystander, close by, but insignificant. This investigation shows that is not the case.
In this approach, with the technical name of Flash-photolysis time-resolved dielectric-loss spectroscopy (fp-TRDL), the reaction sample is irradiated with microwave radiation and by measuring how the microwave changes over time as it passes through the solution the team can quantity how the electrons are shared across the ionic pair. Measurements are taken on the nanosecond scale; one nanosecond is one billionth of a second. Ionic pairs that are strongly bound in solution (two magnets stuck together), produce a strong signal, while an ionic pair that is only weakly bound (two magnets that are separated, but still have some attraction), produce a much weaker signal.
To modernize this technique the RASEI team addressed two critical components. Computer-aided design and simulations were used to fabricate a sample vessel capable of measuring these light-driven reactions, taking measurements in both the ground state (when no light is shining) and in the excited state (when light is being shone on the reaction). And computer code was written capable of analyzing and interpreting the ultra-fast readings taken. This was a great opportunity to showcase the value of this approach, says Garry Rumbles, RASEI Associate Director, and leader of a research thrust in BioLEC.
“Many previous studies with this technique were on fundamental systems, chosen to show how it worked, the chance to work on a catalyst of such current importance was really exciting”.
This revitalized approach provided unprecedented insights into how the catalyst ionic pair behaves in the reaction. Two catalyst systems were investigated, one where the ‘bystander’ anion was small, and the other where the anion was relatively large. When the anion is small, the ionic pair remains tightly bound in the reaction, but when the anion is large, the pair are separated in solution.
What does this mean for the reaction? When the reaction is exposed to light, two different things happen depending on the configuration of the catalyst pair:
- Small anion: With a tightly bound ionic pair, the small anion is an active participant in the transformation. When the catalyst is excited by light the ionic pair reorganizes, stabilizing the excited state. This makes the catalyst effective at donating an electron to other chemicals in the reaction—a reduction transformation.
- Large anion: With a weakly bound ionic pair, the large anion is not available to reorganize in concert with the excited state, so no stabilization is possible, and the catalyst now favors accepting an electron from other chemicals in the reaction—an oxidation transformation.
The measurements show that there is a four-fold difference in selectivity between these oxidation and reduction. Catalysts with a small anion proceed via a reduction pathway and those with a large anion through an oxidation pathway. Being able to control which path a reaction takes is important, not only to make the required products, but also to reduce impurities and waste.
This collaboration, operating at the intersection of cutting-edge light driven catalysis and reinvention of a century-old technique, reveals that molecules which were previously considered inconsequential bystanders can effectively switch the path of a reaction. Before this, organic chemists may have observed that the choice of anion made a difference to their reaction, but with no tools to better understand this, it remained an unexplored observation. This technique provides a valuable tool to investigate these kinds of reactions, and with better understanding comes improved results.
“This is already changing how we think about photoredox reactions in BioLEC” says Rumbles. “The vast promise of light-driven catalysis means that these kinds of detailed insights could pave the way to a greener and more sustainable way of building organic molecules.”


