Length regulation of microtubule antiparallel overlaps by molecular motors
Hui-Shun Kuan and Meredith D. Betterton (2019). bioRxiv DOI:10.1101/743880. Download.
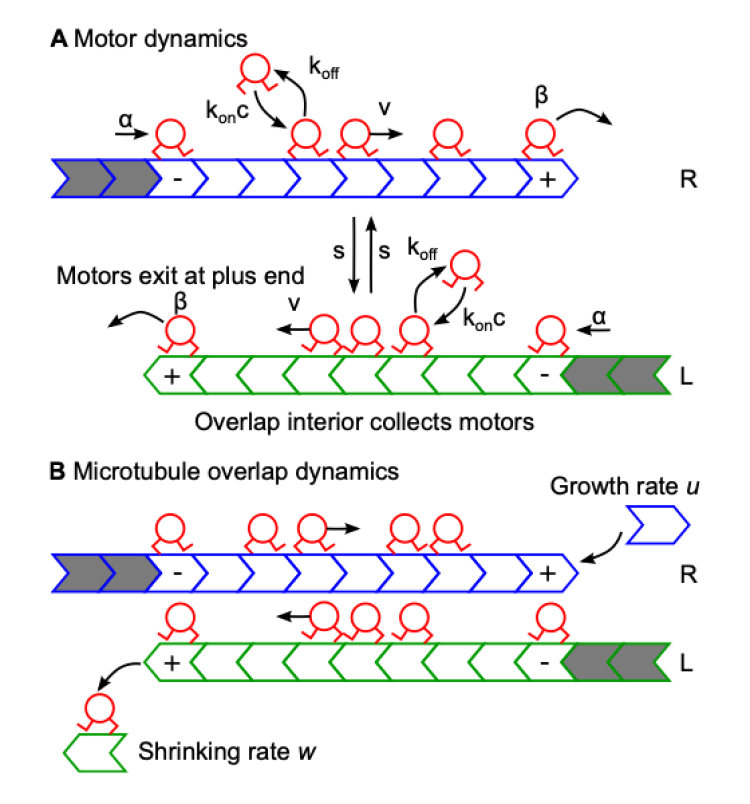
During cell division, microtubules in the mitotic spindle form antiparallel overlaps near the center of the spindle. Kinesin motor proteins alter microtubule polymerization dynamics to regulate the length of these overlaps to maintain spindle integrity. Length regulation of antiparallel overlaps has been reconstituted with purified microtubules, crosslinkers, and motors. Here we develop a theory of steady-state overlap length which depends on the filament plus-end motor concentration, determined by a balance between motor arrival (motor binding and stepping in the overlap) and motor departure (motor unbinding from filament tips during depolymerization) in the absence of motor-driven sliding. Assuming that motors processively depolymerize and exhibit altered binding kinetics near MT plus-ends improves the agreement between theory and experiment. Our theory explains the origin of the experimentally observed critical concentration, a minimum motor concentration to observe a steady-state overlap length.

