Home
Background
The overall interests of the Poyton lab have been on gene expression and cellular enegry production. This has lead us to study the formation and function of the mitochondrion, which is responsible for most of the energy that cells produce and which is dependent on the expression of genes on two different genomes for its formation. During the past 45 years, we have studied mitochondrial biogenesis, mitochondrial genetics, import and export of mitochondrial proteins, crosstalk between the mitochondrial genome (mtDNA) and the nuclear genome (nDNA), mitochondrial control of oxygen-regulated nuclear genes, biophysics of electron transport within mitochondria, cytochrome c oxidase as the regulator of cellular energy production, and mitochondrial production of reactive oxygen species (ROS) and reactive nitrogen species (RNS). We have also discovered that cytochorme c oxidase is an alternative source of cellular nitric oxide, a vasodilator and important intrcellular signalling molecule. More recently, our attention has focussed on the role of mitochondria in aging - an issue of increasing importance as global life expectancy is increasing.
Mitochondria and Aging
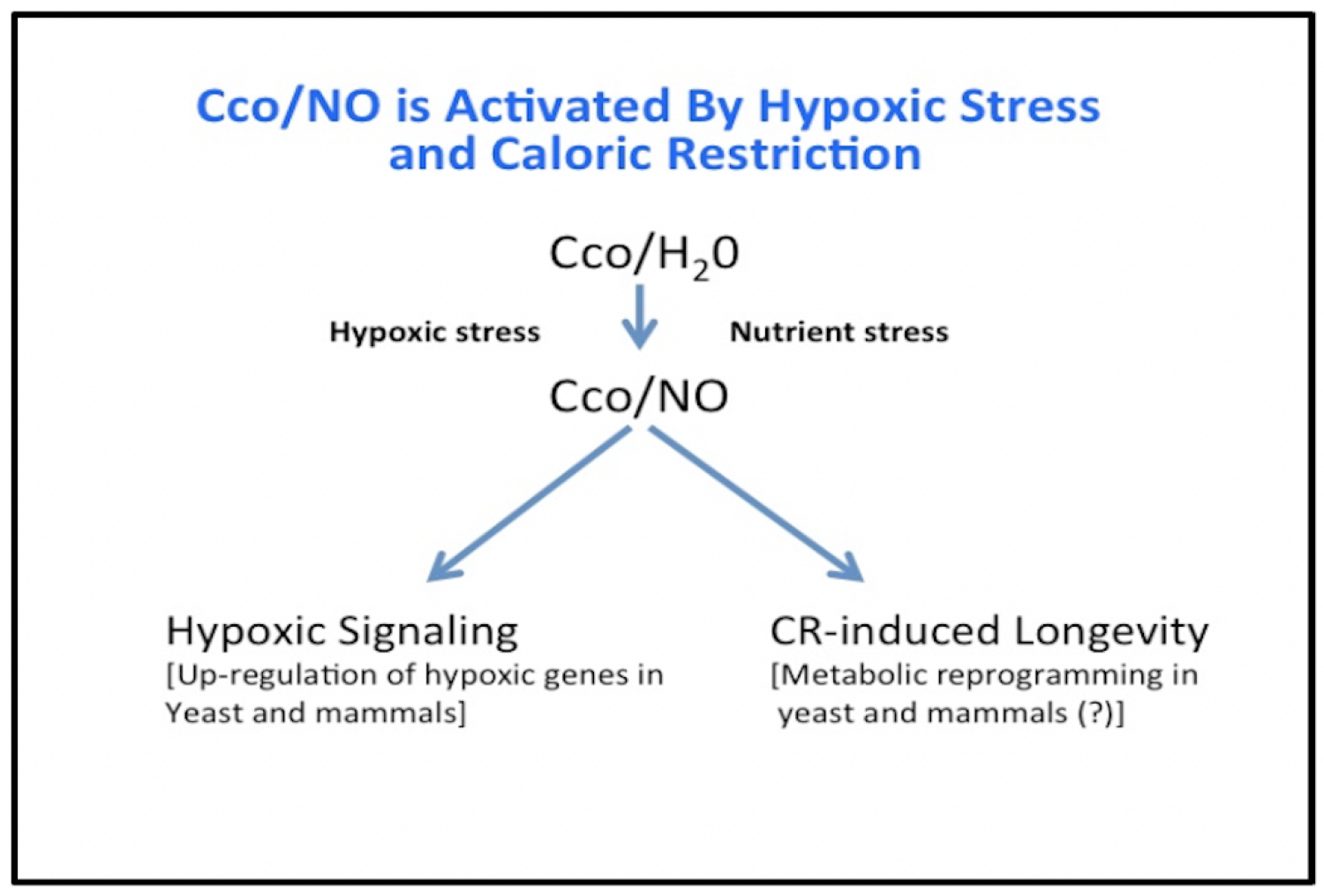
Recent studies have shown that aging starts as a cellular process and then progresses to tissues, organs, and finally to the individual. These studies have also revealed that mitochondria within cells are at the causal epicenter of aging. 'Signals' generated by the mitochondrion as well as the crosstalk between the mitochondrial and nuclear genomes play important roles in aging and in lifespan extension afforded by caloric restriction. Although mitochondria have long been known to be the source for most of the energy generated by eukaryotic cells, recent research has revealed that they play a multitude of additional roles in normal cell function and in the pathophysiologies associated with the degenerative diseases of aging. These functions include: (1) oxygen sensing and hypoxic signalling; (2) generation of free radicals that contribute to aging; (3) lifespan extension that results from limiting caloric intake; (4) inflammation and the development of the inflammasome; (5) initiation of programmed cell death during development; (6) calcium homeostasis; (7) cell growth and division; (8) maintenance of vascular health; and (9) protection against many types of dementia. Most of these roles involve signaling molecules that leave the mitochondrion and exert their effects on metabolism or the expression of nuclear genes. These ‘signals’ include reactive oxygen species, reactive nitrogen species (nitric oxide and peoxynitrite), apoptosis inducing factors, NAD/NADH, ATP, calcium, glutamate, and heme. They may also include small peptides encoded within the ribosomal genes on mtDNA in a pathway that we have named Intragenomic Signaling. As a result of these new findings, it now appears that the mitochondrion is an ‘environmental stress sensor’ within cells that senses and responds to changes in the environment, and that it does so by releasing one or more of the specific signals noted above. It is in this capacity as a 'stress sensor' that the mitochondrion plays a pivotal role in aging. Our laboratory has been focused on the overall role of mitochondrial 'Intragenomic Signaling' in aging, the function of nitric oxide produced by cytochrome c oxidase on the HIF-1α transcription factor and SIRT1 in aging, and the mechanism by which caloric restriction switches cytochrome c oxide from its CcO/H2O to its Cco/NO activity and contibutes to lifespan extension.
Free Radicals and Mitochondrial-Nuclear Crosstalk
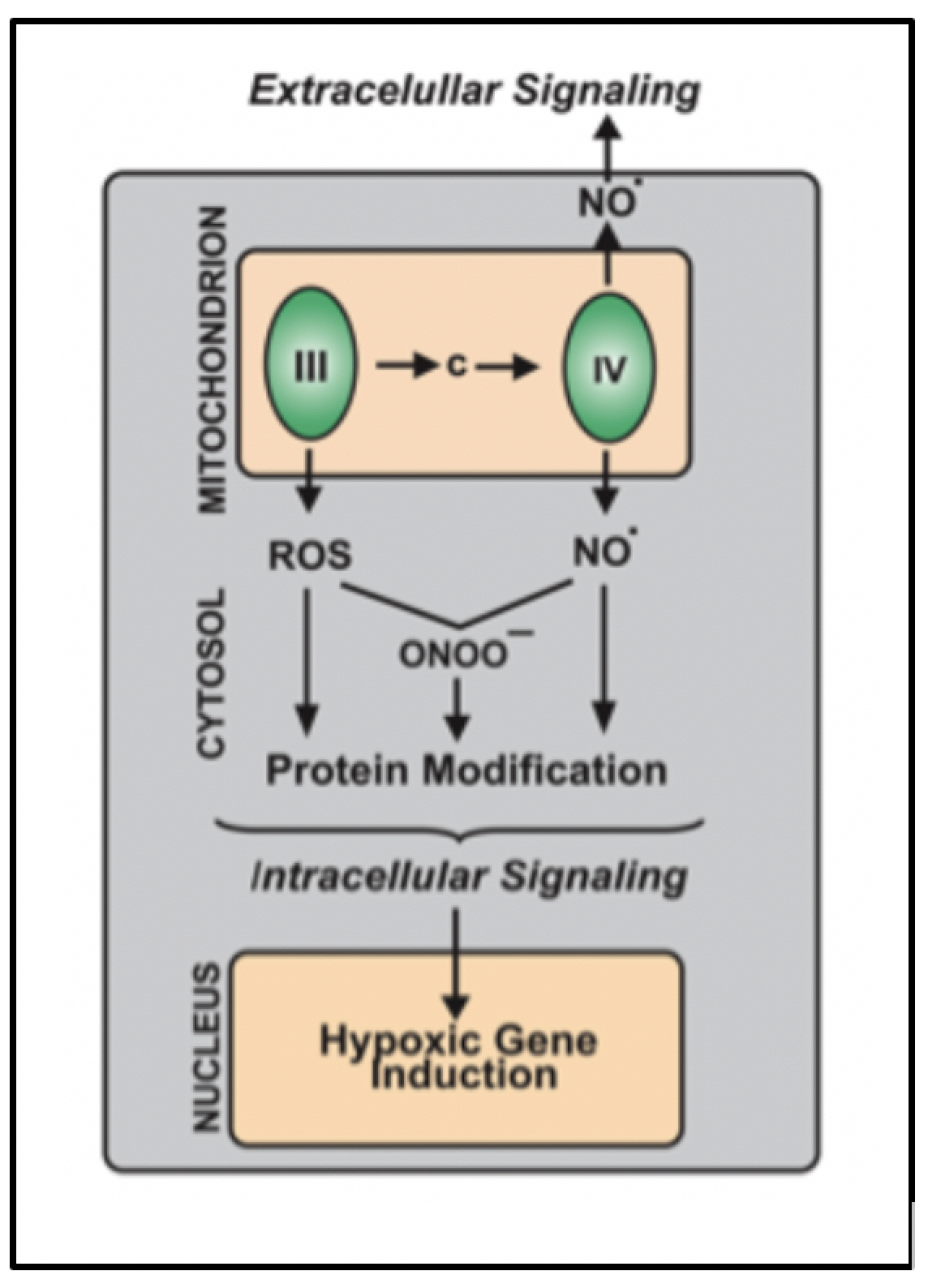
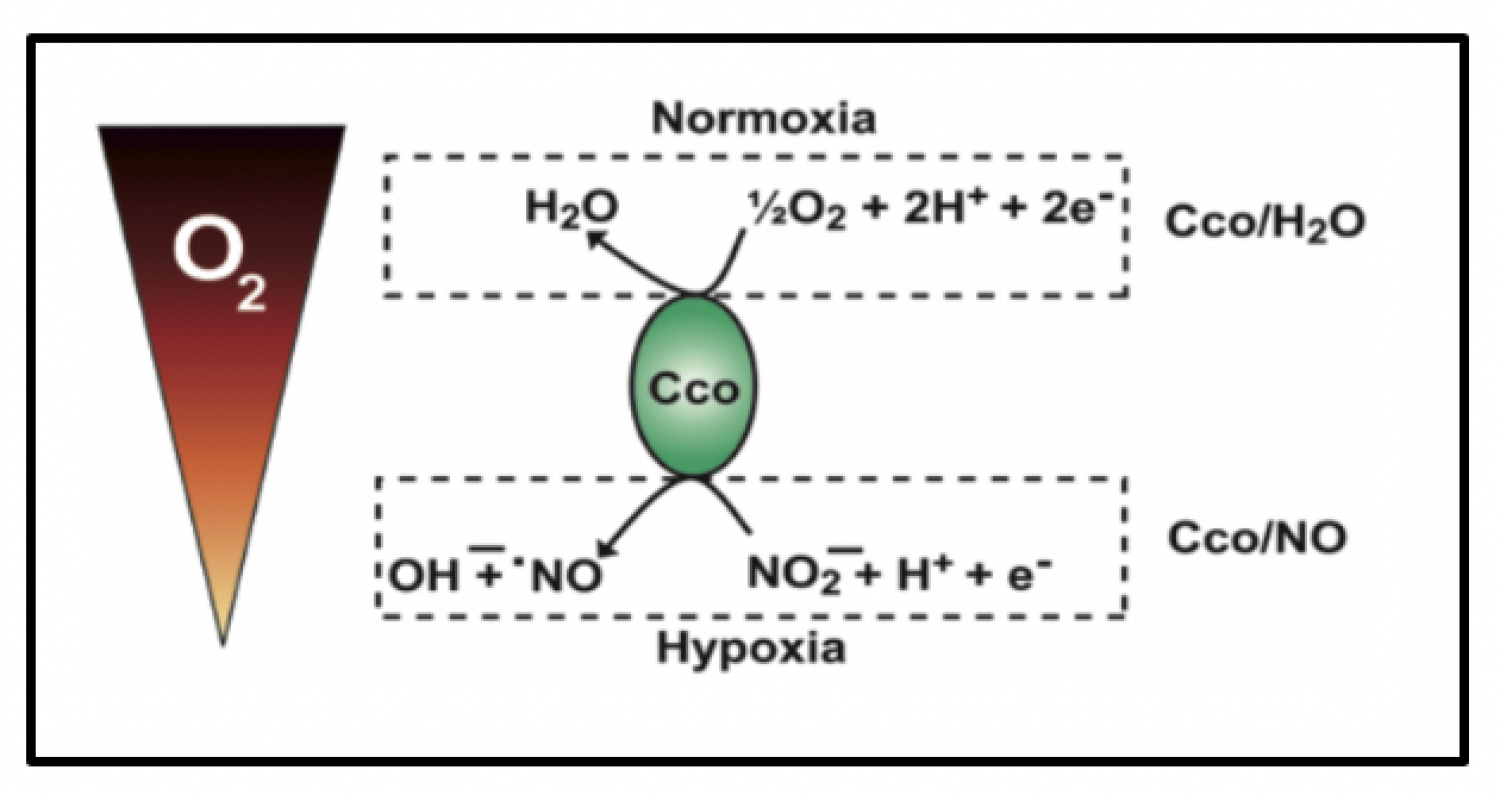
Cytochrome c oxidase is a bifunctional enzyme which functions like a "molecular switch" that responds to the oxygen and nutrient levels that cells experience. In the presence of normal oxygen and nutrient levels, this enzyme catalyzes the reduction of oxygen and hydrogen to water (Cco/H2O), but when levels are low, cytochrome c oxidase reduces nitrite to nitric oxide (Cco/NO). The nitric oxide and/or peroxynitrite produced by the mitochondria interacts with at least two proteins (SIRT1 and HIF-1α) that are involved in aging. SIRT1 is a NAD-dependent protein deacetylase that has been implicated in both aging and dementias. HIF-1α is a transcription factor that functions to upregulate a large number of human genes when oxygen concentrations drop. As such, it plays a role in hypoxia and aging.
Caloric Restriction