Motor guidance by long-range communication through the microtubule highway
Sithara S. Wijeratne*, Shane A. Fiorenza*, Radhika Subramanian, and Meredith D. Betterton (2022). biorXiv. DOI: 10.1101/2020.12.23.424221. PNAS 119, e2120193119 DOI: 10.1073/pnas.2120193119. Download.
*Equal contribution
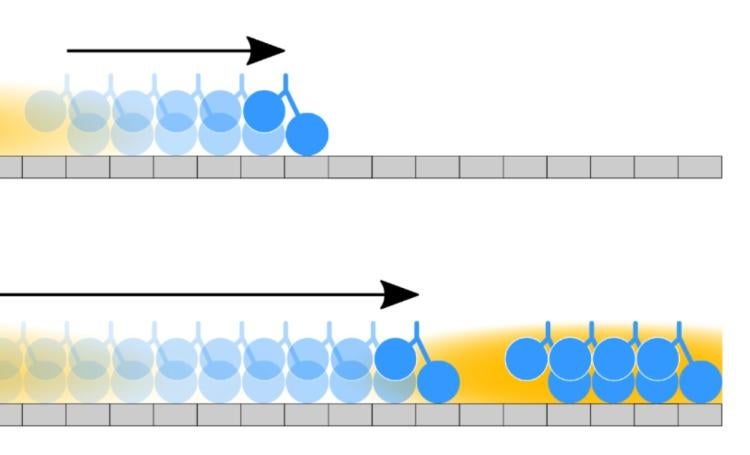
Coupling of motor proteins within arrays drives muscle contraction, flagellar beating, chromosome segregation, and other biological processes. Current models of motor coupling invoke either direct mechanical linkage or protein crowding, which rely on short-range motor-motor interactions. In contrast, coupling mechanisms that act at longer length scales remain largely unexplored. Here we report that microtubules can physically couple motor movement in the absence of short-range interactions. The human kinesin-4 Kif4A changes the run-length and velocity of other motors on the same microtubule in the dilute binding limit, when 10-nm-sized motors are separated by microns. This effect does not depend on specific motor-motor interactions because similar changes in Kif4A motility are induced by kinesin-1 motors. A micron-scale attractive interaction potential between motors is sufficient to recreate the experimental results in a computational model. Unexpectedly, our theory suggests that long-range microtubule-mediated coupling not only affects binding kinetics but also motor mechanochemistry. Therefore, motors can sense and respond to motors bound several microns away on a microtubule. These results suggest a paradigm in which the microtubule lattice, rather than being merely a passive track, is a dynamic medium responsive to binding proteins to enable new forms of collective motor behavior.

