Contributions of microtubule dynamic instability and rotational diffusion to kinetochore capture
Robert Blackwell, Oliver Sweezy-Schindler, Christopher Edelmaier, Zachary R. Gergely, Patrick J. Flynn, Salvador Montes, Ammon Crapo, Alireza Doostan, J. Richard McIntosh, Matthew A. Glaser, and Meredith D. Betterton (2017). Biophysical Journal 112, 552-563. DOI: 10.1016/j.bpj.2016.09.006 arXiv DOI: 1606.07847. Download. Highlighted article on the Biophysical Journal web page.
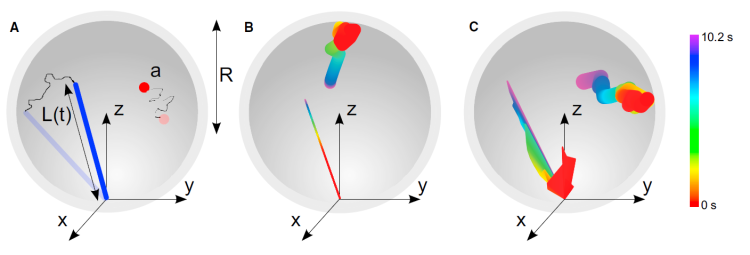
Microtubule dynamic instability allows search and capture of kinetochores during spindle formation, an important process for accurate chromosome segregation during cell division. Recent work has found that microtubule rotational diffusion about minus-end attachment points contributes to kinetochore capture in fission yeast, but the relative contributions of dynamic instability and rotational diffusion are not well understood. We have developed a biophysical model of kinetochore capture in small fission-yeast nuclei using hybrid Brownian dynamics/kinetic Monte Carlo simulation techniques. With this model, we have studied the importance of dynamic instability and microtubule rotational diffusion for kinetochore capture, both to the lateral surface of a microtubule and at or near its end. Over a range of biologically relevant parameters, microtubule rotational diffusion decreased capture time, but made a relatively small contribution compared to dynamic instability. At most, rotational diffusion reduced capture time by 25%. Our results suggest that while microtubule rotational diffusion can speed up kinetochore capture, it is unlikely to be the dominant physical mechanism. In addition, we found that when microtubules undergo dynamic instability, lateral captures predominate even in the absence of rotational diffusion. Counterintuitively, adding rotational diffusion to a dynamic microtubule increases the probability of end-on capture.

