Background: In addition to the development of stoichiometric reagents, we are interested in the development of selective catalysts for organic synthesis. Our work has focused on two areas, the design of new acyl transfer catalysts, and the synthesis of asymmetric ligands for transition metals. Our work on acyl transfer catalysis has focused on the design on new catalytic cycles and on mechanistic studies to aid in the design of new catalysts, while our approach to the synthesis of chiral ligands took on a more empirical flavor.
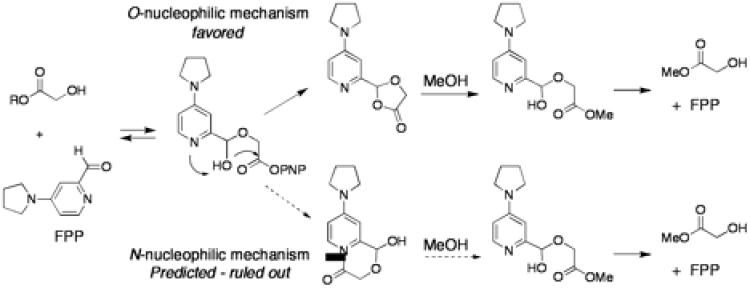 Hydroxyl-Directed Acyl Transfer Catalysis: Our research on acyl transfer catalysis began with an interest in rendering ester hydrolysis hydroxyl-directed, and thereby enable the selective hydrolysis of α-hydroxy esters. We wished to design a catalyst that contained two sites, one for binding to a hydroxyl group, and another nearby reactive site, and settled on an aldehyde as the binding site and the 4-amino pyridine scaffold as the reactive site. Binding of the hydroxyl group to the aldehyde (by reversible formation of a hemiacetal) would bring the ester close to the pyridine nitrogen, and enable nucleophilic catalysis and hydrolysis at a faster rate than the background reaction. This idea worked, but not by the mechanism we designed; rather than nucleophilic catalysis by the pyridine nitrogen, we observed general base catalysis by the nitrogen, and nucleophilic catalysis by the hydroxyl group of the hemiacetal (formed upon addition of the hydroxyl group of the substrate to the aldehyde).
Hydroxyl-Directed Acyl Transfer Catalysis: Our research on acyl transfer catalysis began with an interest in rendering ester hydrolysis hydroxyl-directed, and thereby enable the selective hydrolysis of α-hydroxy esters. We wished to design a catalyst that contained two sites, one for binding to a hydroxyl group, and another nearby reactive site, and settled on an aldehyde as the binding site and the 4-amino pyridine scaffold as the reactive site. Binding of the hydroxyl group to the aldehyde (by reversible formation of a hemiacetal) would bring the ester close to the pyridine nitrogen, and enable nucleophilic catalysis and hydrolysis at a faster rate than the background reaction. This idea worked, but not by the mechanism we designed; rather than nucleophilic catalysis by the pyridine nitrogen, we observed general base catalysis by the nitrogen, and nucleophilic catalysis by the hydroxyl group of the hemiacetal (formed upon addition of the hydroxyl group of the substrate to the aldehyde).
 Our mechanistic work allowed us to design catalysts with remarkable selectivity for the cleavage of α-hydroxy esters over α-alkoxy esters with relative rates of reaction exceeding 2,000:1. A list of catalysts and their relative rates and selectivities are provided above.
Our mechanistic work allowed us to design catalysts with remarkable selectivity for the cleavage of α-hydroxy esters over α-alkoxy esters with relative rates of reaction exceeding 2,000:1. A list of catalysts and their relative rates and selectivities are provided above.
References:
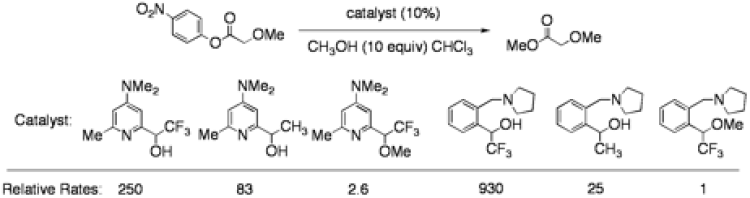 Asymmetric Hydrolysis of α-Amino and α-Acetoxy Esters: During our mechanistic work on hydroxyl-direct ester hydrolysis, we observed an undirected background reaction that appeared to proceed by an O-nucleophilic mechanism wherein a hemiacetal hydroxyl group was acting as a nucleophile, with concomitant deprotonation by a proximal base. We hypothesized that the increased basicity of the hemiacetal hydroxyl group facilitated catalysis, and designed a series of catalysts bearing hydroxyl groups of varying acidity to test this hypothesis. We found that, in fact, catalysts bearing more acidic hydroxyl groups were more active, and confirmed the O-nucleophilic nature of our catalysts with mechanistic studies.
Asymmetric Hydrolysis of α-Amino and α-Acetoxy Esters: During our mechanistic work on hydroxyl-direct ester hydrolysis, we observed an undirected background reaction that appeared to proceed by an O-nucleophilic mechanism wherein a hemiacetal hydroxyl group was acting as a nucleophile, with concomitant deprotonation by a proximal base. We hypothesized that the increased basicity of the hemiacetal hydroxyl group facilitated catalysis, and designed a series of catalysts bearing hydroxyl groups of varying acidity to test this hypothesis. We found that, in fact, catalysts bearing more acidic hydroxyl groups were more active, and confirmed the O-nucleophilic nature of our catalysts with mechanistic studies.
 All of the catalysts described above are chiral (though they were prepared as racemic mixtures), and we wished to study asymmetric ester cleavage with chiral esters. After much experimentation, we found that catalyst 7, shown in the scheme below, is capable of the enantioselective hydrolysis of α-acetoxy and α-amido esters with excellent selectivity. The catalyst can be prepared in three steps the inexpensive starting material, sec-phenethylamine.
All of the catalysts described above are chiral (though they were prepared as racemic mixtures), and we wished to study asymmetric ester cleavage with chiral esters. After much experimentation, we found that catalyst 7, shown in the scheme below, is capable of the enantioselective hydrolysis of α-acetoxy and α-amido esters with excellent selectivity. The catalyst can be prepared in three steps the inexpensive starting material, sec-phenethylamine.
References:
Notte, G. T.; Sammakia, T. "Kinetic Resolution of Protected a-Amino Acid Derivatives by a Chiral O-Nucleophilic Acyl Transfer Catalyst" J. Am. Chem. Soc.2006,128, 4230 – 4231.
Notte, G. T.; Sammakia, T. "Kinetic Resolution of a-Acetoxy N-Acyl Oxazolidinethiones by a Chiral O-Nucleophilic Acyl Transfer Catalyst" J. Am. Chem. Soc.2005, 127,13502 – 13503.
Oxocarbenium Ions in Organic Synthesis
Methods and Strategies for the Total Synthesis of the Oxo Polyene Macrolide Antibiotics
